Difference between revisions of "DuoFertility"
(media) |
(formatting) |
||
| Line 48: | Line 48: | ||
== Important Dates == | == Important Dates == | ||
| − | 2005 - Cambridge Temperature Concepts founded.<ref name="businesswire" /> | + | * 2005 - Cambridge Temperature Concepts founded.<ref name="businesswire" /> |
| − | January 2009 - The device obtains European Medical Devices Directive approval.<ref name="businesswire" /> | + | * January 2009 - The device obtains European Medical Devices Directive approval.<ref name="businesswire" /> |
| − | December 2011 - DuoFertility receives FDA clearance for the US market.<ref>http://mobihealthnews.com/16028/duofertility-wireless-sensor-receives-fda-clearance/</ref> | + | * December 2011 - DuoFertility receives FDA clearance for the US market.<ref>http://mobihealthnews.com/16028/duofertility-wireless-sensor-receives-fda-clearance/</ref> |
| − | April 2012 - Commercial launch for the United States.<ref name="mobihealth" /> | + | * April 2012 - Commercial launch for the United States.<ref name="mobihealth" /> |
== Enhancement/Therapy/Treatment == | == Enhancement/Therapy/Treatment == | ||
Revision as of 09:43, 5 August 2016
| DuoFertility | |
|---|---|
|
|
| Category | Torso-mounted |
| Developer | Cambridge Temperature Concepts Ltd [1] |
| Announced | 2005 [2] |
| Released | Developers:
Consumers: 2012 [3] |
| Price | 440 USD (May 2016, price for the 3 month plan)[4] |
| Operating system | none |
| Sensors |
temperature [5] |
| Weight | g (negligible) |
| Controls |
buttons, smartphone, desktop |
| Data available | Robust |
| Risk factor | Low |
| Not Standalone | |
| https://www.duofertility.com | |
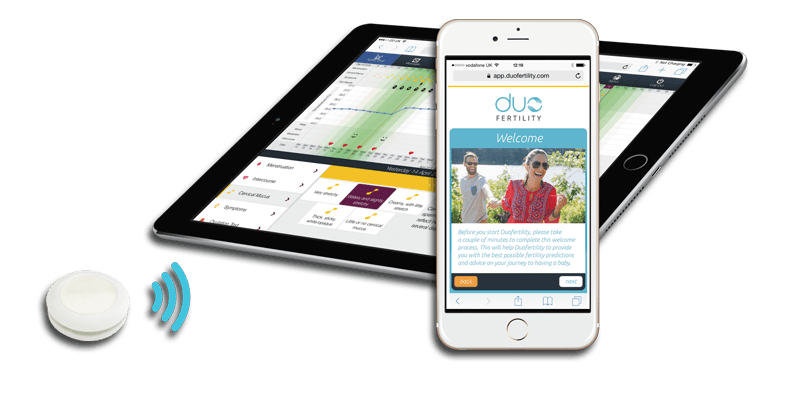
DuoFertility is a wearable temperature sensor that measures the changes in body temperature which occur during ovulation. The goal of the manufacturer is to help women with conception since they will be now able to monitor when they are the most fertile. The company claims a success rate of conception that is comparable to in vitro fertilisation.
Contents
Main characteristics
The device consists of two separate pieces, the body temperature monitor, and the processing unit that wirelessly connects to the provided mobile app.
DuoFertility temperature sensors constantly monitors the changes in body temperature and sends the data to be analysed and stored in the processing unit. The user can also log various aspects of their menstruation cycle. All the gathered data are analysed and can be viewed in the provided mobile app. The user can make the decision to conceive based on the data however the company also provides support from fertility specialists as a part of the purchase. These are able to access and interpret the data and can offer advice on when will be the best time to conceive.
Purpose
The purpose of the device is to help pair with infertility by analysing the data about the women's ovulation and offering advice on when is the best time to conceive.
Company & People
DuoFertility is developed by a British company Cambridge Temperature Concepts Ltd. The company is based in Cambridge and was established in 2005. It focuses on the development of continuous physiological monitoring devices.[6]
Important Dates
- 2005 - Cambridge Temperature Concepts founded.[2]
- January 2009 - The device obtains European Medical Devices Directive approval.[2]
- December 2011 - DuoFertility receives FDA clearance for the US market.[7]
- April 2012 - Commercial launch for the United States.[3]
Enhancement/Therapy/Treatment
Enhancement. - DuoFertility helps couples to better predict the timespanhttps://www.duofertility.com/clinically-proven/ when the women is the most fertile and thus enhances their ability to conceive.
Ethical & Health Issues
Public & Media Impact and Presentation
There are numerous articles about DuoFertility to be found, as well as user reviews and discussions.
Mobihealthnews has several articles about DuoFertility.[8] The first one discusses the presentation of DuoFertility at the Wireless Life-Sciences Alliance in San Diego in 2011. The article stated that the device was among the surprising presentation during that event and that it "really does span the continuum of health care" thanks to its incorporation of wireless technology.[9] Another articles informs about the device receiving the approval of the US Food and Drug Administration and that it took almost two years to receive.[10]
Public Policy
We are not aware of any policy that is regulating or is otherwise relevant to this device in particular.
Related Technologies, Projects or Scientific Research
In a pilot study with 8 infertile women, DuoFertility was able to identify ovulation with 100% accuracy within one day.[15] A cohort study with 200 couples revealed that the success rate of conception assisted by DuoFertility is statistically comparable to in vitro fertilisation.[16]
https://www.duofertility.com/clinically-proven/
References
- ↑ https://www.duofertility.com/about-us/contact/
- ↑ 2.0 2.1 2.2 http://www.businesswire.com/news/home/20090107005556/en/Cambridge-Temperature-Concepts-gains-medical-approval-revolutionary
- ↑ 3.0 3.1 http://mobihealthnews.com/17150/duofertility-commercially-launches-in-us-for-795
- ↑ https://www.duofertility.com/buy/
- ↑ https://www.duofertility.com/what-is-duofertility/your-guide-to-using-duofertility/
- ↑ http://www.temperatureconcepts.com/
- ↑ http://mobihealthnews.com/16028/duofertility-wireless-sensor-receives-fda-clearance/
- ↑ http://mobihealthnews.com/tag/duofertility
- ↑ http://mobihealthnews.com/11042/wireless-health-opportunity-begins-at-conception
- ↑ http://mobihealthnews.com/16028/duofertility-wireless-sensor-receives-fda-clearance
- ↑ http://mobihealthnews.com/tag/cambridge-temperature-concepts
- ↑ http://mobihealthnews.com/17150/duofertility-commercially-launches-in-us-for-795
- ↑ http://techcrunch.com/2013/04/01/duofertility/
- ↑ http://www.telegraph.co.uk/news/health/news/8069642/British-fertility-device-as-effective-as-IVF.html
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4106957/
- ↑ http://www.sciencedirect.com/science/article/pii/S1472648310625448