Difference between revisions of "Thync"
(correction of categories) |
|||
| Line 161: | Line 161: | ||
[[Category:External Hardware or Software]] | [[Category:External Hardware or Software]] | ||
| − | [[Category:Electronic and Other | + | [[Category:Electronic and Other devices]] |
| − | |||
| − | |||
| − | |||
[[Category:Brain Stimulation]] | [[Category:Brain Stimulation]] | ||
| + | [[Category:Transcranial direct-current stimulation]] | ||
Revision as of 09:08, 23 September 2016
| Thync | |
|---|---|
|
|
| Category | Other Head-mounted Devices |
| Developer | Thync |
| Announced | October 2014 |
| Released | |
| Price | 299 USD (2015) |
| Operating system | iOS 8 |
| Sensors | Measuring brain waves |
| Weight | 18 g |
| Controls | the Thync App on the compatible iOS 8 devices (iPhone/iPod). Android App coming in 2015 |
| Data available | Robust |
| Risk factor | Low |
| Not standalone[1] | |
| http://www.thync.com/ | |
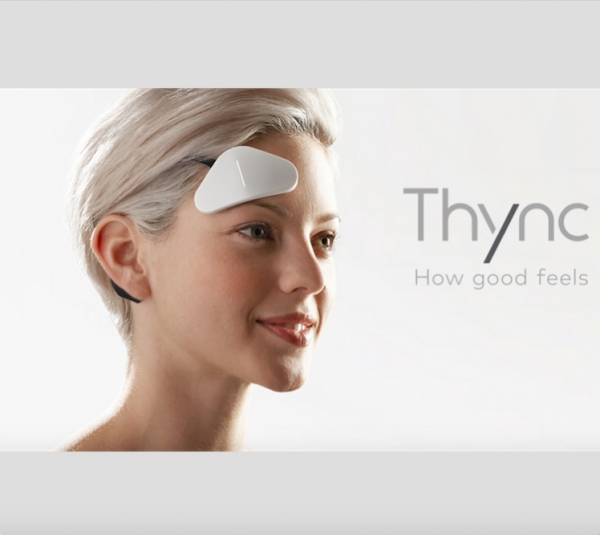
Thync is a small triangular-shaped head-mounted device that puts on the wearer's head. Headset stimulates and activates nerves, well, the user can either relax or energize. Brain neurons remain intact, the device releases only electrical impulses. In this way, Thync controls user´s mood..[2]
Thync is wireless, connected to the smartphone or tablet via Bluetooth (iOS or Android app). Users can control Thync by the official app where they can choose the length of the session. Also, they can adjust the strength of the brain-zapping there (each program follows a pattern of greater and lesser intensity, with cycles of peaks and valleys, but they can also manually raise or lower the overall strength).[3]
Company Thync raised for this project $13.000.000 and started from October 2014”[2]. From 02.06.2015 is the device already publicly available and its price is 299$ (7 245,37 CZK to the 22.9.2016)).”[4]
Contents
Main characteristics
Thync releases the low-level electrical pulses to the nerves in the regions of brain. In this way, change of mood is occurs. The producer describes strictly, how to change the mood occurs: "Human body balances the activity between sympathetic and parasympathetic nervous systems. The sympathetic system is associated with a "fight or flight" response to help regulate his reaction to stress. The parasympathetic system counteracts stress to help his enter a relaxed "rest and digest" mode".[5]
Thync uses neurosignaling to the change of mood. Neurosignaling is on their website defined as follows: "Neurosignaling is the coupling of an energy waveform to a neural structure (receptor, nerve or brain tissue) to modulate its activity. Neurosignaling waveforms or Vibes consist of precise algorithms that bias activity of the sympathetic and parasympathetic nervous systems, so that you can enjoy a shift into a more energetic or relaxed state. Neurosignaling builds upon the best features of long-standing tDCS and TENS techniques by using pulsed currents with lower-intensity and higher-frequency outputs delivered through bio-compatible materials for greater safety and comfort."”[5]
Purpose
Thync is a wearable device whose main purpose is to change the mood. Users can choose 2 modes: calm or energy. One session takes 15 minutes.
Company & People
- Isy Goldwasser - CEO and Founder
- Jamie Tyler Ph.D. - CSO and Founder
- Sumon Pal Ph.D. - Chief of Vibes
- Anil Thakur - CTO
- Jason Egnal - VP, Digital Marketing & Commercial Operations[6]
Important Dates
- 2011 - Co-founding of Company Thync by experts in the fields of neurobiology, neuroscience and consumer electronics from institutions that include MIT, Harvard, and Stanford Universities.
- October 2014 - Beginning of the project Thync.
- June 2015 - start selling the device Thync.”[6]
Ethical Issues
So far there are known no ethical issues. In the future, but some may occur because it wasn't tested the long-term use of the device and it was only in 2015 released to the public.
Health Risks
According to he company, there have been no significant issues regarding Thync’s safety profile. It's known that many people engage in alcohol, drugs, and other activities due to stress, anxiety, and mood problems. Thync may allow for a safer way to alleviate these problems.[7]
Thync commented safety of their products like this: "The Thync System is a low-risk transdermal neurostimulation device intended for lifestyle use at home, work, or in wellness applications to temporarily induce mental relaxation or calmness or to temporarily increase energy, awareness, and alertness. The Thync system is a safe and low-risk device. It is not intended to treat or diagnose any disease or medical condition."”[5]
As already stated above, the Thync uses TENS and TDCs techniques. "Transcutaneous electrical nerve stimulation(TENS)is a non-invasive analgesic technique that is used to relieve nociceptive, neuropathic, and musculoskeletal pain."[8] Transcranial direct current stimulation (TDCS) is characterized as "the most widely publically-marketed kind of brain stimulation device for cognitive enhancement."”[9]
Risk of these methods is wrong placement of electrodes. Some people are right-handed, but the others are left-handed. Reversing the polarity can be dangerous and can lead to impairment of brain. At the best, it could mean ineffectiveness, in the worst, headache and brain disorders.”[9] The manufacter this device recommends that people suffering to Reflex Syncope should be consult their physician before purchasing a Thync System.”[5]
Enhancement/Therapy/Treatment
In addition to the device changes mood, it could help with sleep problems, reduce stress or the motivation to exercise. Producers also believe that the people could reduce consumption of coffee, alcohol and drugs, as a result of introduction this device.[10]
It should be noted that Thync is not intended to treat or diagnose any disease or medical condition. Thync is only enhancement device.”[5]
Public & Media Impact and Presentation
Apart from the fact, that the Thync company has its own website, facebook, twitter and youtube channel, there are the another web portals talking about Thync. One example of this is web portal Quora.com. From example, Yates Buckeley here wrote:
"When released as a product Thync was explained to be a high frequency current stimulation. Research in this area is new so the mechanism (like most brain things) is not well understood."[11]
The most frequently raised points of confusion:
- most people find it relaxing
- some do not / has some side effects
- not well understood
- long term use unclear
- not a medical device”[11]
Yates also points out that the recent study found a slight decrease in IQ as a result of basic electrical stimulation and he is afraid of a possible associating with Thync:
"I presume somehow it reset some connections. I have a slight concern Thync could have a similar effect but this is very hard to test."”[10]
TODO: http://www.digitaltrends.com/fitness-apparel-reviews/thync-review/
Public Policy
The FDA notified that with regard to its use and output characteristics, Thync is not subject to medical device regulations requiring pre-market clearance or approval.”[5]
Related Technologies, Project or Scientific Research
Already, Thync has conducted studies with hundreds participants, and their chief science officer Jamie Tyler is the leading researcher in the neuromodulation (with publications in Nature, PLoS ONE, Neuron, and Brain Stimulation). From the perspective of MedTech Boston, their responses were substantially better than those given by many other neurotech companies touting their wares on the convention floor of CES.[12]
The company Thync published 2 studies that are concerned with their product. First study was called "The tolerability of transcranial electrical stimulation used across extended periods in a naturalistic context by healthy individuals.". This study examined the safety and tolerability of device. The research involved 100 healthy individuals (63% males and 37% female). It was conducted and observed in the total of 1905 treatment sessions (sham= 636, tDCS= 623, and tPCS= 646) on a total of 100 subjects (sham= 37, tDCS= 33, and tPCS= 30). No severe adverse events were reported. Between or during the sessions, atypical discomfort, headache or migraine or skin condition occurred rarely .[13]
The second study was called "Transdermal neuromodulation of noradrenergic activity suppresses psychophysiological and biochemical stress responses in humans" and it tested the impact of Neurosignaling on participants. The study is very extensive, therefore, I write only the results. Data showed that "TEN can significantly dampen basal sympathetic tone compared to sham in a manner sufficient to modulate emotional thermoregulation as reflected in temperature changes of the face."[14]
References
- ↑ Shows if the device is a standalone wearable computer or if it needs to be connected to a processing unit to function.
- ↑ 2.0 2.1 ADHIKARI, Richard. TECHNEWSWORLD. Thync Scores $13M for Foggy Brain Project[online]. Copyright 1998-2016 ECT News Network. [retr. 22.09.2016]. Available online at: http://www.technewsworld.com/story/81165.html
- ↑ GIZMAC.COM. Thync mood-changing wearable officially launches - we go hands on (again) [online]. All content copyright © Gizmag 2003 - 2015 [retr. 16.10.2015]. Available online at: http://www.gizmag.com/thync-hands-on-2/37820/
- ↑ THYNC.COM. Thync Launches First Wearable to Shift Your State of Mind [online]. Copyright 2016 Thync [retr. 22.09.2016]. Available online at: http://www.thync.com/resources/press-release/thync-launches-first-wearable-to-shift-your-state-of-mind
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 THYNC.COM. Science/Technology [online]. Copyright 2015 Thync [retr. 20.10.2015]. Available online at: http://www.thync.com/science-and-technology
- ↑ 6.0 6.1 THYNC.COM. About Us [online]. Copyright 2015 Thync [retr. 16.10.2015]. Available online at: http://www.thync.com/about
- ↑ MEDTECH BOSTON. Testing Thync: A Calming, Energizing Personal Brain Modulator [online]. ©2013-2016 Medical Networking, Inc. [retr. 8.11.2015]. Available online at: https://medtechboston.medstro.com/testing-thync-a-calming-energizing-personal-brain-modulator/
- ↑ JOHNSON, Mark. Transcutaneous electrical nerve stimulation [online]. Oxfords Journals, 2009. [retr. 10.12.2015]. Available online at: http://ceaccp.oxfordjournals.org/content/early/2009/06/26/bjaceaccp.mkp021.full.pdf+html
- ↑ 9.0 9.1 MALEN, Hannah. DOUGLAS, Thomas. KADOSH, Roicohen. LEVY, Neil. SAVULESCU, Julian. Mind Machines. Universtity of Oxford, 2014. [retr. 10.12.2015]. Available online at: http://www.oxfordmartin.ox.ac.uk/downloads/briefings/Mind_Machines.pdf.
- ↑ 10.0 10.1 TECHKRUNCH. Hands-On With Thync's Mood-Altering Headset [online]. © 2013-2015 TechCrunch. [retr. 9.12.2015]. Available online at: http://techcrunch.com/2015/06/02/hands-on-with-thyncs-mood-altering-headset/#.fdgpwg:JNRc
- ↑ 11.0 11.1 QUORA.COM. What are the potential dangers of brain-zapping devices like Thync? [online]. Quora. com [retr. 8.11.2015]. Available online at: https://www.quora.com/What-are-the-potential-dangers-of-brain-zapping-devices-like-Thync
- ↑ MEDTECH BOSTON. Testing Thync: A calming, energizing personal brain modulator [online]. MedTech Boston Medical ©2013-2016 [retr. 8.12.2015]. Available online at: https://medtechboston.medstro.com/testing-thync-a-calming-energizing-personal-brain-modulator/
- ↑ PANERI, Bhascar. The tolerability of transcranial electrical stimulation used across extended periods in a naturalistic context by healthy individuals. [online]. New York : The City College of New York, 2015. [retr. 10.12.2015]. Available online at: http://cdn2.hubspot.net/hubfs/432410/documents/peerJ.pdf?t=1447979598033
- ↑ TYLER, Wiliam J. BOASSO, Alyssa M. MORTIMORE, Hailey M. SILVA, Rhonda S. CHARLESWORTH, Jonathan D. MARLIN, Michelle A. AEBERSOLD, Kirsten. AVEN, Linh. WETMORE, Daniel Z. SUMON, K. Pal. Transdermal neuromodulation of noradrenergic activity suppresses psychophysiological and biochemical stress responses in humans [online]. Boston : Scientific reports, 2015. ISSN 2045-2322 [retr. 10.12.2015]. Available online at: http://www.nature.com/articles/srep13865#references