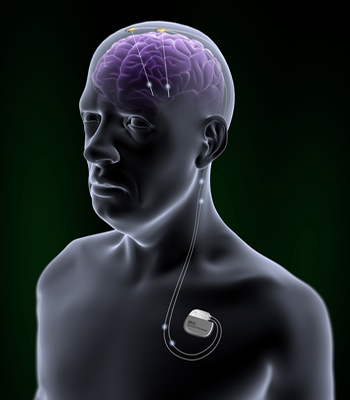
Brio™ Rechargeable IPG
| Brio™ Rechargeable IPG | |
|---|---|

|
|
| Category | Deep brain stimulation |
| Developer | St. Jude Medical, Inc. [1] |
| Announced | 2008 [2] |
| Released | Developers:
Consumers: 2008 [1] |
| Price | 19 150 USD [3] |
| Max output | 12.512.5 T 12.5 mA 0.0125 A mA[2] |
| Session duration | 0.050.05 s 8.333334e-4 minute s each pulse, the current is delivered constantly[2] |
| Scalp location | subthalamic nucleus, globus pallidus internus, ventral intermediate nucleus [4][5][6] |
| Weight | 29 g [1] |
| Controls |
Brio™ Patient Programmer, magnet [6] |
| Data available | |
| Risk factor | |
| Medical prescription | yes |
| https://www.sjmglobal.com/en-int/professionals/featured-products/neuromodulation/deep-brain-stimulation/implantable-pulse-generators/brio-ipg | |
Brio™ Rechargeable IPG (Implantable Pulse Generator) provides deep brain stimulation therapy for patients suffering by Parkinson disease and essential tremor. It does not cure the diseases but it could help to reduce their symptoms. It is used primarily in the cases when the medication of the disease is not successful.[7]
The device was developed by St. Jude Medical, Inc. the company which focus on the medical devices and is settled in St. Paul, Minnesota.[8]
Contents
Main Characteristics
Brio™ Rechargeable IPG was developed as a medical device which could provide treatment through deep brain stimulation. It is a standalone device and could be received only on prescription. It has to be implanted only on special clinics and the quality of surgery has a huge impact on the results of the treatment.[6] The device was approved as a treatment of symptoms of Parkinson disease, essential tremor,[9] and dystonia.[10]
The device consists of:
- Implantable pulse generator (IPG)
- Extensions
- Leads
- Patient programmer
- Patient magnet
- Charging system
The leads are implanted into the brain in accordance with the purposed treatment. The IPG is implanted on the upper part of the chest and could be controlled by patent programmer. Magnet could turn on or of the IPG.[6]
The IPG provides a long term brain stimulation. It contains a rechargeable battery. The device could be charged via antenna.[11] Brio™ Rechargeable IPG is a system which operate open-loop. It means that the IPG delivers continuous stimulation to patient's brain.[12] In addition, Brio™ Rechargeable IPG provides a constant-current stimulation, which means that the IPG deliver constant current to the brain. It is contrasted with voltage-controlled stimulation, in which the amount of affected tissue may vary.[5]
Purpose
The main purpose of the device is to treat the symptoms of certain incurable diseases and improve the patients' life in this way.
Company & People
The device was originally manufactured by St. Jude Medical, Inc, the company from St. Paul, Minnesota.[9] This company was however acquired by Abbott Laboratories in 2017.[13] Abbott Laboratories seats in Abbott Park, Illinois.[14]
- Miles D. White - Chairman and Chief Executive Officer of Abbott Laboratories
- Hubert L. Allen - Executive Vice President, General Counsel and Secretary of Abbott Laboratories[15]
- Michael T. Rousseau - President, Cardiovascular and Neuromodulation of Abbott Laboratories, former CEO of St. Jude Medical, Inc.[16]
Important Dates
- 1976 - the company St. Jude Medical, Inc. was founded.[17]
- 2009 - Brio™ Rechargeable IPG received CE (Conformité Européenne) mark of approval for treatment of Parkinson disease.[9]
- 2010 - Brio™ Rechargeable IPG was approved by the Australian Therapeutic Goods Administration (TGA).[8]
- 2013 - Brio™ Rechargeable IPG received CE (Conformité Européenne) mark of approval for treatment of Dystonia symptoms.[10]
- 2015 - Brio™ Rechargeable IPG was approved by FDA.[7]
- 2017 - St. Jude Medical, Inc. was acquired by Abbott Laboratories.[13]
Enhancement/Therapy/Treatment
Brio™ Rechargeable IPG is a medical device, which was developed as a treatment of symptoms of Parkinson's disease, Essential tremor[9] and Dystonia[10]. It is not meant for cognitive enhancement, but the possibility of certain cognitive enhancement has also not been excluded yet.
Parkinson's disease
Parkinson's disease is neurodegenerative disease. It is caused by the loss of brain cells which produce dopamine.[18] Its symptoms could be resting tremor, muscular rigidity, bradykinesia, and postural instability.[19] The symptoms appear gradually and the progression of the disease differs among patients. Parkinson's disease affects primarily the population over 60. The disease in incurable at present[18], but a variety of medications help to relief the symptoms. Levodopa combined with carbidopa may helps relief bradykinesia and rigidity, bromocriptine, pramipexole and ropinirole mimic the role of dopamine and appear to reduce mentioned symptoms, anticholinergic drugs may help control tremor.
Brio™ Rechargeable IPG is one of deep brain stimulation devices which could treat the symptoms of Parkinson's disease. The suggested treatment consists in bilateral stimulation of the subthalamic nucleus. This treatment focuses on the patients which disease cannot be controlled by medications.[6]
Essential Tremor
Essential tremor is a movement disorder, when one or more parts of patient's body (particularly upper limbs) move unintentionally. It appears mainly among population over 60. The movement is rhythmic and the affected parts oscillate.[20] It could be non-progressive but it is usually progressive. The disease is widespread primarily among population over 60.[12] There has not been developed the medication especially for the treatment of essential tremor. Certain drugs are used off-label to the treatment, but approximately 50% of patients cannot tolerated any medication or the symptoms persist even after the medication. These patients could be treated by lesional therapies or brain stimulation,[12] if the illness possess a significant functional disability.[6]
Brio™ Rechargeable IPG treat the essential tremor by the unilateral or bilateral stimulation of the ventral intermediate nucleus, which is a part of thalamus.[6]
Dystonia
Dystonia is a movement disorder, which consists in an involuntary movement as the contraction of muscles, repetitive movements, spasms, etc. The movement is often painful. The primarily or idiopathic dystonia is not caused by the injury or disease and might be inherited.[21] The disorder may be also caused by other factors (secondary dystonia) such as birth-related or other trauma, infection or reaction to pharmaceutical drugs (e.g. neuroleptics) [10].
There is no treatment which could be effective in the treatment of all patients with dystonia.[21] The therapy response among patients is very individual. The treatment includes pharmacological intervention, physical therapy and other supportive therapies. Various treatments focus on sedating brain functions by medications such as anticholinergics (e.g. diphenhydramine, benzatropine and atropine), anti-Parkinsons agents (e.g. ropinirole and bromocriptine), muscle relaxants (e.g. diazepam). A different therapeutic approach is blocking nerve communications with the muscles via drugs, neuro-suppression, or denervation by botulin toxin injection into affected muscles. When the pharmacotherapy is not successful, patients could be treated also with deep brain stimulation. In the case of dystonia, the stimulation of Globus pallidus or the ventral intermedius nucleus seems to be more efficient.[10]
It is possible that Brio™ Rechargeable IPG could be used as a treatment of other neurological diseases as neuropathic pain,[22] anorexia nervosa,[23] or Alzheimer's disease[24], but it has not been approved as a treatment of this diseases yet.
Ethical & Health Issues
Although Brio™ Rechargeable IPG could bring relief patients suffering Parkinson's disease, essential tremor and dystonia, there are many health issues linked with the device. They are identified in "Brio™ Patient Programmer: User's Guide"[6] or the report provided by FDA.[2]
The first important issue is the selection of suitable patients. The patient should be able to handle with the device and the programmer. Additionally, the doctor should test the patient carefully, in order to decide if this treatment is suitable for the disease the patient is suffering. Certain medical examinations and therapies such as electroshock therapy, transcranial magnetic stimulation, diathermy, magnetic resonance, external defibrillators, and therapeutic radiation could also negatively interfere with the device. The device could also negatively interfere with other devices as implanted peacemaker, microwave, mobile phones, theft detectors, metal screening devices, appliances containing magnet etc. The device should not be also implanted to pregnant or nursing women.[6]
Several issues are linked with the surgery. The complications as haemorrhage or cerebrospinal fluid leakage could appear during surgery. The main complication after surgery is infection. After the surgery, patients also reported side-effects as dysarthria, fatigue, postoperative pain and discomfort, paraesthesias, and oedema.[4] Patients could also struggle with the depression and suicidal intentions or attempts.[6]
The everyday use of the device could also arise certain issues. Patients are warned not to use extensive stimulation and charge density, since the high amplitudes and wide pulses could damage their tissue. The low frequency, in contrast, could cause tremor. Since the device includes rechargeable battery, the device has to be charged regularly. The charging could be painful or uncomfortable for patients, because the device heated during the charging.[6] However, the rechargeable battery significantly reduces the number of surgeries and the use of a rechargeable device is cheaper than the use of a non-rechargeable device. Therefore, the rechargeable devices as Brio™ Rechargeable IPG are favoured by certain researchers.[25] Patients could also encounter issues linked with the stimulation itself as: speech or language impairment including, aphasia, dysphagia, dysarthria, and hypophonia, supranuclear gaze palsy, hypersexuality or increased libido, or worsening existing medical conditions.[6]
It was reported a case of a series of Brio™ Rechargeable IPG, where the IPG ceased delivering the stimulation. It was caused by the faulty battery component, which prevent charging of the device and communication with the programmer. St. Jude Medical, Inc. identified the problem and sent "Field Safety Notice" to the doctors, with the serial numbers of the devices which might be affected by this failure. St. Jude Medical, Inc. offered a replacement of the devices affected by the failure which had not been implanted and those which had been implanted and failed to deliver the stimulation. In other cases, they recommended a careful monitoring of the patients, which have implanted devices which could contain faulty battery.[26] There were also reported 11 cases in which the leakage of body fluids interrupted therapy. St. Jude Medical, Inc. pulled off all devices which were not implanted and warn the doctors as in the previous case. They recommended the use of Libra™.[27]
Public & Media Impact and Presentation
The newspaper refers about the approval of Brio™ Rechargeable IPG as the new possibility of the treatment of incurable diseases' symptoms:
"There are no cures for Parkinson's disease or essential tremor, but finding better ways to manage symptoms is essential for patients," says Dr. William Maisel of the FDA. "This new device adds to the array of treatment options to help people living with Parkinson's and essential tremor enjoy better, more productive lives."[28]
Maggie McGuire Kuhl from "The Michael J. Fox Foundation" cited Michael Okun, MD, professor of neurology, who believe that the more devices in the market will be beneficial for patients:
"The Brio System’s approval is good news for the Parkinson’s patient community because the availability of another device and another company tin the market will stimulate more rapid development of DBS technology."[29]
The newspapers' articles also mentioned that Brio™ Rechargeable IPG is significantly small and therefore could be more comfortable for patients.[1][30]
Michele Tagliati, MD, Director of the Movement Disorders Program in the Department of Neurology at Cedars-Sinai Medical Center, claims for "Practical Neurology":
"Particularly in Parkinson’s disease we can be very successful for many years” managing symptoms medically, Dr. Tagliati observed. DBS is used when medical treatment is no longer sufficient. Use of DBS can be beneficial once the proper settings are in place. “In the short term we have to work, sometimes pretty hard, to find the electrical settings that will stop the tremor, that will help the patients relieve their stiffness or their slowness of movement[.]”[31]
Fenna T. Phibbs, MD, MPH, Assistant Professor of Neurology at Vanderbilt University School of Medicine in Nashville, points out in "Neurology Reviews" certain issue:
"A potential difficulty of the Brio system is that insurance companies have been reluctant to cover systems with rechargeable batteries[.]"[32]
Public Policy
The medical use of Brio™ Rechargeable IPG was approved by CE[9], TGA[8], FDA[7] for Parkinson's disease and essential tremor and by CE[10] for the treatment of dystonia.
St. Jude Medical, Inc. owns several patents, which are listed on their web sites.[33] They, however, apply also on other devices developed by St. Jude Medical, Inc. The patents which are clearly associated with Brio™ Rechargeable IPG are:
- Brio™ clinician programmer - U.S. Patent No. 7228179
- Brio™ IPG 16-channel rechargeable - U.S. Patent Nos. 7180760; 7228179; 7751879
- Brio™ patient controller - U.S. Patent No. 7228179[33]
Related Technologies, Projects, or Scientific Research
St. Jude Medical, Inc. developed also two other deep brain stimulation devices Libra™ and Infinity™ DBS IPG.
Brio™ Rechargeable IPG was examined in several papers. There are two studies conducted by St. Jude Medical:
- St. Jude Medical. Parkinson’s Study. Final Report. 2012. n = 136
- St. Jude Medical. Tremor Study Final Report. 2014. n = 127
It was also used in various studies:
- BOČEK, Václav et al. Pallidal stimulation in dystonia affects cortical but not spinal inhibitory mechanisms. Journal of the Neurological Sciences, 2016, 369, 19–26.
- FERNÁNDEZ-PAJARÍN G. et al. Bilateral pallidal deep brain stimulation in myoclonus-dystonia: our experience in three cases and their follow-up. Acta Neurochirurgica, 2016, 158(10), 2023–2028.
- GILLIES, Martin J. et al. Rechargeable vs. Nonrechargeable Internal Pulse Generators in the Management of Dystonia. Neuromodulation: Technology at the Neural Interface, 2013, 16(3), 226-229.
- GRAY, Alan M. et al. Deep Brain Stimulation as a Treatment for Neuropathic Pain: A Longitudinal Study Addressing Neuropsychological Outcomes. The Journal of Pain, 2014, 15(3), 283–292.
- KRAUSE, P. et al. Long-term results of deep brain stimulation in a cohort of eight children with isolated dystonia. Journal of Neurology, 2016, 263(11), 2319–2326.
- OKUN, M. et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. The Lancet Neurology, 2012, 11(2), 140-149.
- PREDA, F. et al. Switching from constant voltage to constant current in deep brain stimulation: a multicenter experience of mixed implants for movement disorders. European Journal of Neurology, 2016, 23(1), 190–195.
- WANG, Jing et al. Coordinated Reset Deep Brain Stimulation of Subthalamic Nucleus Produces Long-Lasting, Dose-Dependent Motor Improvements in the 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine Non-Human Primate Model of Parkinsonism. Brain Stimulation, 2016, 9(4), 609–617.
References
- ↑ 1.0 1.1 1.2 1.3 St. Jude Medical. St. Jude Medical Receives CE Mark Approval for World’s Smallest, Longest-Lasting Rechargeable Deep Brain Stimulator for Parkinson’s Disease. Newswise, Inc [online]. 2009, Sep 9. Available online at: http://www.newswise.com/articles/st-jude-medical-receives-ce-mark-approval-for-world-s-smallest-longest-lasting-rechargeable-deep-brain-stimulator-for-parkinson-s-disease-first-patient-implanted-with-brio-neurostimulator-at-university-of-cologne-germany32 (Retrieved 6th April, 2017).
- ↑ 2.0 2.1 2.2 2.3 U.S. Food and Drug Administration [online]. Summary of Safety and Effectiveness Data. U.S. Food and Drug Administration [online]. Available online at: https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140009b.pdf (Retrieved 11th April, 2017).
- ↑ Australian Government. Private Health Insurance (Prostheses) Rules 2015 (No. 1). Federal Register of Legislation [online]. Available online at: https://www.legislation.gov.au/Details/F2015C00649/Html/Volume_2 (Retrieved 6th April, 2017).
- ↑ 4.0 4.1 OKUN, M. et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. The Lancet Neurology, 2012, 11(2), 140-149. Doi: 10.1016/S1474-4422(11)70308-8 Available online at: http://www.sciencedirect.com/science/article/pii/S1474442211703088 (Retrieved 6th April, 2017).
- ↑ 5.0 5.1 PREDA, F. et al. Switching from constant voltage to constant current in deep brain stimulation: a multicenter experience of mixed implants for movement disorders. European Journal of Neurology, 2016, 23(1), 190–195. Doi:10.1111/ene.12835 Available online at: http://onlinelibrary.wiley.com/doi/10.1111/ene.12835/full (Retrieved 6th April, 2017).
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 St. Jude Medical. Brio™ Patient Programmer. U.S. Food and Drug Administration [online]. Available online at: www.accessdata.fda.gov/cdrh_docs/pdf14/P140009c.pdf (Retrieved 6th April, 2017).
- ↑ 7.0 7.1 7.2 U.S. Food and Drug Administration. FDA approves brain implant to help reduce Parkinson’s disease and essential tremor symptoms. U.S. Food and Drug Administration [online]. 2015, Jun 12. Available online at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm451152.htm (Retrieved 6th April, 2017).
- ↑ 8.0 8.1 8.2 St. Jude Medical. St. Jude Medical's Brio DBS system for treating Parkinson’s disease receives Australian TGA approval. News-Medical.Net [online]. 2010, Mar 23. Available online at: http://www.news-medical.net/news/20100323/St-Jude-Medicals-Brio-DBS-system-for-treating-Parkinsone28099s-disease-receives-Australian-TGA-approval.aspx (Retrieved 6th April, 2017).
- ↑ 9.0 9.1 9.2 9.3 9.4 BUSINESS WIRE. St. Jude Medical Receives CE Mark Approval for World’s Smallest, Longest-Lasting Rechargeable Deep Brain Stimulator for Parkinson’s Disease. BUSINESS WIRE [online]. 2009, Sep 9. Available online at: http://www.businesswire.com/news/home/20090909005095/en/St.-Jude-Medical-Receives-CE-Mark-Approval (Retrieved 6th April, 2017).
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 GUILLIOTTI, Kimberly. St Jude Medical’s Deep Brain Stimulation Trifecta: New Treatment Options for Dystonia in EU. MD Buyline [online]. 2013, Apr 23. Available online at: http://www.mdbuyline.com/st-jude-medicals-deep-brain-stimulation-trifecta-new-treatment-options-for-dystonia-in-eu/ (Retrieved 6th April, 2017).
- ↑ St. Jude Medical, Inc. Brio™ Rechargeable IPG: Advanced Technology. St. Jude Medical, Inc. [online]. Available online at: https://www.sjmglobal.com/en-int/professionals/featured-products/neuromodulation/deep-brain-stimulation/implantable-pulse-generators/brio-ipg?halert=show&clset=92f57278-460e-4300-b7fe-89e52a04194f%3acadddb93-fcc4-47f2-8ceb-fd88f01ca17f (Retrieved 7th April, 2017).
- ↑ 12.0 12.1 12.2 PICILLO, Marina, FASANO, Alfonso. Recent advances in Essential Tremor: Surgical treatment. Parkinsonism & Related Disorders, 2016, 22(1), S171–S175. DOI: 10.1016/j.parkreldis.2015.09.012 Available online at: http://www.sciencedirect.com/science/article/pii/S1353802015003879 (Retrieved 6th April, 2017).
- ↑ 13.0 13.1 St. Jude Medical, Inc. Abbott Acquisition of St. Jude Medical Set to Close on January 4, 2017. Jude Medical, Inc. [online]. 2016, Dec 30. Available online at: http://media.sjm.com/newsroom/news-releases/news-releases-details/2016/Abbott-Acquisition-of-St-Jude-Medical-Set-to-Close-on-January-4-2017/default.aspx (Retrieved 7th April, 2017).
- ↑ Abbott Laboratories. Contacts. Abbott Laboratories [online]. Available online at: http://www.abbott.com/contact.html (Retrieved 7th April, 2017).
- ↑ Abbott Laboratories. Executive Team. Abbott Laboratories [online]. Available online at: http://www.abbott.com/corpnewsroom/utilities/executive-team.html (Retrieved 7th April, 2017).
- ↑ Abbott Laboratories. Michael T. Rousseau. Abbott Laboratories [online]. Available online at: http://www.abbott.com/corpnewsroom/utilities/executive-team/michael-rousseau.html (Retrieved 7th April, 2017).
- ↑ St. Jude Medical, Inc. Our History: Investing in People and Ideas. St. Jude Medical, Inc. [online]. Available online at: https://www.sjmglobal.com/en-int/about/company-facts/history?clset=92f57278-460e-4300-b7fe-89e52a04194f%3acadddb93-fcc4-47f2-8ceb-fd88f01ca17f (Retrieved 6th April, 2017).
- ↑ 18.0 18.1 National Institute of Neurological Disorders and Stroke. Parkinson's Disease Information Page. National Institute of Neurological Disorders and Stroke [online]. Available online at: https://www.ninds.nih.gov/Disorders/All-Disorders/Parkinsons-Disease-Information-Page (Retrieved 10th April, 2017).
- ↑ POLYMEROPOULOS, M. H. et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science [online]. 1997, Jun 27. 276(5321), 2045-2047. Available online at: http://ve5kj6kj8s.scholar.serialssolutions.com/?sid=google&auinit=MH&aulast=Polymeropoulos&atitle=Mutation+in+the+%CE%B1-synuclein+gene+identified+in+families+with+Parkinson%27s+disease&id=pmid:9197268 (Retrieved 10th April, 2017).
- ↑ National Institute of Neurological Disorders and Stroke. Essential Tremor Information Page. National Institute of Neurological Disorders and Stroke [online]. Available online at: https://www.ninds.nih.gov/Disorders/All-Disorders/Essential-Tremor-Information-Page (Retrieved 10th April, 2017).
- ↑ 21.0 21.1 National Institute of Neurological Disorders and Stroke. Dystonias Information Page. National Institute of Neurological Disorders and Stroke [online]. Available online at: https://www.ninds.nih.gov/Disorders/All-Disorders/Dystonias-Information-Page (Retrieved 10th April, 2017).
- ↑ GRAY, Alan M. et al. Deep Brain Stimulation as a Treatment for Neuropathic Pain: A Longitudinal Study Addressing Neuropsychological Outcomes. The Journal of Pain, 2014, 15(3), 283–292. Doi: 10.1016/j.jpain.2013.11.003 Available online at: http://www.sciencedirect.com/science/article/pii/S1526590013013849 (Retrieved 12th April, 2017).
- ↑ LOZANO, Andres M. Deep brain stimulation of the subcallosal cingulate area for treatment of refractory anorexia nervosa. United States Patent [online]. Available online at: http://patft.uspto.gov/netacgi/nph-Parser?Sect2=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/search-bool.html&r=1&f=G&l=50&d=PALL&RefSrch=yes&Query=PN/9446238 (Retrieved 12th April, 2017).
- ↑ DE RIDDER, Dirk. Methods for Treating Mild Cognitive Impairment and Alzheimer's Disease. US Patent & Trademark Office [online]. Available online at: http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=/netahtml/PTO/srchnum.html&r=1&f=G&l=50&s1=20150290460.PGNR. (Retrieved 12th April, 2017).
- ↑ GILLIES, Martin J. et al. Rechargeable vs. Nonrechargeable Internal Pulse Generators in the Management of Dystonia. Neuromodulation: Technology at the Neural Interface, 2013, 16(3), 226-229. Doi: 10.1111/ner.12026 Available online at: http://onlinelibrary.wiley.com/doi/10.1111/ner.12026/full (Retrieved 11th April, 2017).
- ↑ St. Jude Medical, Inc. Important Medical Device Information. Gov.uk [online]. 2011, May 24. Available online at: https://assets.publishing.service.gov.uk/media/5485ac15e5274a42900002ab/con117559.pdf (Retrieved 11th April, 2017).
- ↑ MassDevice Staff. St. Jude yanks Brio deep brain stimulator. MassDevice [online]. 2012, Mar 29. Available online at: http://www.massdevice.com/st-jude-yanks-brio-deep-brain-stimulator/ (Retrieved 11th April, 2017).
- ↑ MCINTOSH, James. Parkinson's brain implant approved by FDA. Medical News Today [online]. 2015, Jun 15. Available online at: http://www.medicalnewstoday.com/articles/295376.php?trendmd-shared=0 (Retrieved 7th April, 2017).
- ↑ MCGUIRE KUHL, Maggie. FDA Approves New Deep Brain Stimulation Device. FoxFeed Blog [online]. 2015, Jun 16. Available online at: https://www.michaeljfox.org/foundation/news-detail.php?fda-approves-new-deep-brain-stimulation-device (Retrieved 11th April, 2017).
- ↑ Medgadget. St. Jude’s Brio Neurostimulator for Parkinson’s, Essential Tremor FDA Approved. Medgadget [online]. 2015, Jun 16th. Available online at: http://www.medgadget.com/2015/06/st-judes-brio-neurostimulator-for-parkinsons-essential-tremor-fda-approved.html (Retrieved 11th April, 2017).
- ↑ Practical Neurology. New Option Now Available for DBS for PD, Tremor. Practical Neurology [online]. 2015, Jun. Available online at: http://practicalneurology.com/2015/06/new-option-now-available-for-dbs-for-pd-tremor/ (Retrieved 11th April, 2017).
- ↑ GREB, Erik. What Is the Future of DBS for Movement Disorders? Neurology Reviews. 2015, Nov, 23(11): 48. Available online at: http://www.mdedge.com/neurologyreviews/article/104119/movement-disorders/what-future-dbs-movement-disorders (Retrieved 11th April, 2017).
- ↑ 33.0 33.1 St. Jude Medical, Inc. Neuromodulation Patent Information. St. Jude Medical, Inc. [online]. Available online at: https://www.sjmglobal.com/en-int/legal-notices-patents/patents/neuromodulation-patents (Retrieved 12th April, 2017).