Transcranial magnetic stimulation
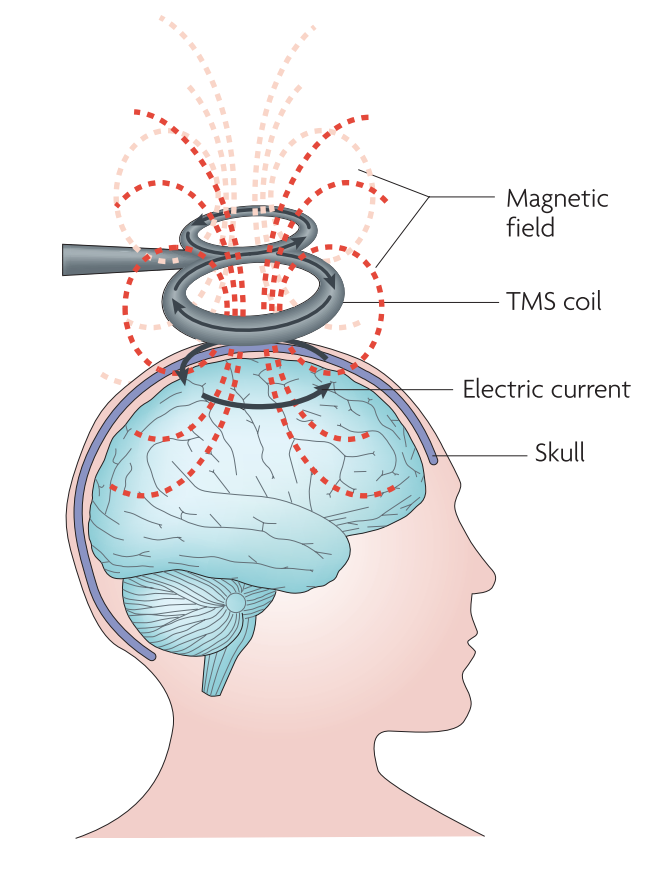
Transcranial magnetic stimulation (TMS), is a non-invasive method of brain stimulation. During the procedure, a magnetic coil is placed over the scalp of the person receiving the treatment. The magnetic field penetrates the skull and the electric stimulus activates the neurons in the target area. This can be used for exploring how the brain works by disrupting its normal behaviour with the stimulation, or, in the case of repetitive transcranial magnetic stimulation (rTMS), for modulation the brain activity for therapeutic purposes.[1]
Magnetic stimulation is used for mapping the human brain, mainly the primary motor cortex because the reaction of the body to the stimulus is easily observable. This is how the location of brain functions of the brain were localized, first for the motor functions, by measuring the motor evoked potentials in the muscles, and now it is possible to localize cognitive and sensory processes with TMS as well.[2]
rTMS is recommended as the clinical treatment method for depression, auditory hallucinations and possibly for negative symptoms of schizophrenia.[3] Evidence-base guidelines on the therapeutic use of rTMS[4] reccommend this method also for the treatment of epilepsy, tinnitus, motor stroke, Parkinson´s disease and neuropatic pain. There exists also studies, which confirmed the effect of TMS in the treatment of migraine[5]. Currently, a number of rTMS devices such as rTMS System from Magstim[6], MagVita system from Magventure[7], NeuroStar TMS Therapy system from Neuronetics, Inc.,[8] and Brainsway Deep TMS system[9] have been approved by the U.S. Food and Drug Administration for the treatment of depression[1]
Contents
Main Characteristics
The magnetic field created by the coil produces electrical current in the brain of the subject due to the electromagnetic induction effect. This produces secondary ionic current which activates the near-surface neuronal axons in the target area. However, TMS, especially in higher intensities, may activate any neuron along the way.[10] The magnetic field can reach up to 2 T and usually lasts for about 100 µs.[2]
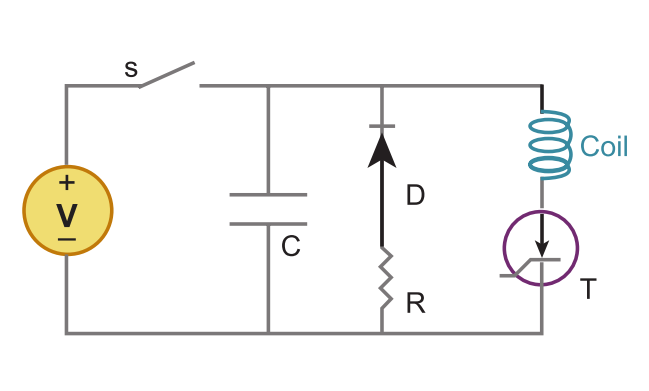
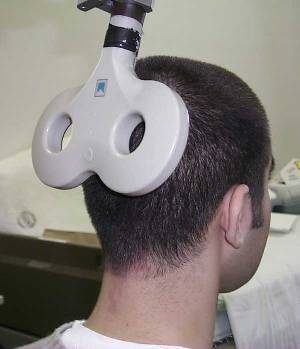
TMS system is composed of two parts. A high-voltage charge-discharge component that produces the required current waveform. This is a RLC circuit modified so it produces the waveform with the lowest amount of heat generated. The second components is the current carrying coil that servers as the emitter of the magnetic field. Although the design of the coils vary, the most used are two circular coil in parallel so as they resemble the number eight, or butterfly, shape. The coils are made of copper wiring and enclosed in a plastic chassis, with the diameter ranging from 4 to 9 centimetres.[11][12] The range of the magnetic field from the centre of the coil is about two centimetres, therefore the stimulation is applied to the surface areas such as brain cortex and not to deeper brain areas e.g. to basal ganglia.
Generally, rTMS is used in two different protocols: low frequency (1 Hz and less) and high frequency (usually 10 Hz and more). While low frequency protocols facilitate cortical excitability, high frequency rTMS leads to the inhibition of cortical excitability. There exists also the modification of high frequency rTMS called theta burst stimulation (TBS), which seems to produce greater changes in cortical excitability than those observed in conventional rTMS protocols in some cases.
Purpose
Transcranial magnetic stimulation was traditionally used for brain mapping, especially of the motor cortex. The repetitive form of TMS (rTMS) has been also demonstrated as a therapeutic tool. It can remedy patients suffering from major depression. Other conditions which have been effectively treated by TMS includes auditory hallucinations in schizophrenia and neuropathic pain. It can be probably used in the treatment of negative symptoms of schizophrenia, migraine, tinnitus, epilepsy, anorexia nervosa or Alzheimer's disease. It could also help in post-stroke rehabilitation.
It could be also used to enhance cognitive abilities (memory, motor reaction speed, verbal fluency) in healthy individuals, although the efficacy of these effects is disputed in the literature.
Historical overview
The physical principles behind magnetic field induction generating electrical current in nearby conductors were discovered by Michael Faraday in 1881. The experimentation with the effects of electricity on the human body begun in the 18th century and continued until the beginning of the 20th century. In 1937, electroconvulsive therapy (ECT) was developed by the Italians Cerletti and Bini. The procedure was originally used to treat schizophrenia but became so popular it was used in virtually every psychological treatment. This resulted in a series of unwanted side-effects and the public stop tolerating the use of the technique. In 1976, the U.S. Food and Drug Administration assumed regulatory control over these types of devices.[13]
Late 1970s saw the creation of the Transcranial direct-current stimulation that was, together with TMS, beginning to be considered for diagnostic and therapeutic purposes among physicians. In years 1984 - 1985 Anthony Barker and his colleagues manufactured first TMS stimulator.[13] One of the first papers to utilize TMS was published in 1991.[14] The authors used repetitive TMS (rTMS) to induce speech difficulties in patients while counting. Other study claimed that TMS can be successfully used in the treatment of depression in 1995.[15] The late 1990s meant wide adoption of TMS into practice, although the treatment was, and in general still is, used as a form of last resort after more established techniques fail.[16] A consensus conference in Italy in 2008 re-evaluated the guidelines for TMS from 1998. In October 2008, the U.S. Food and Drug Administration (FDA) approved the NeuroStar TMS Therapy system from Neuronetics, Inc. to be used in the industry-sponsored clinical trial of the treatment of medication-refractory depression (FDA approval K061053) through TMS.[17] In 2013, Cerena device was approved by FDA as a treatment of pain associated with migraine headache with aura. This device use single-pulse TMS (sTMS).[18]
Important Dates
- 1831 - Michael Faraday discovered electromagnetic induction on which TMS is based.
- 1937 - The ECT was presented by Cerletti and Bini.
- 1984/1985 - The first TMS stimulator was manufactured by Anthony Barker and his colleagues.[13]
- 1991 - The publication of one of the first papers, which describes research, which utilized rTMS.[14]
- 1995 - The publication of the study, which focuses on the treatment of depression by TMS.[15]
- 1998 - TMS risk and safety guidelines were published by Eric M. Wassermann and Sarah H Lisanby.[16]
- 2008 - The consensus conference which review the guideline published 10 years ago took place in Italy.[13]
- October 2008 - NeuroStar TMS system was approved by FDA as a treatment of medication-refractory depression.[17]
- 13th December 2013 - Cerena was classified into class II. devices and approved for the treatment of pain associated with migraine headache with aura.[18]
Enhancement/Therapy/Treatment
TMS represents a noninvasive method used in diagnostics of neurological disorders and in physiological research of sensory and motor functions and intracortical relations. Repetitive form, rTMS has primary role in treatment of neuropsychiatric symptoms.
Evidence-base guidelines on the therapeutic use of rTMS[4] reccommend this method for the treatment of depression, auditory hallucinations and negative symptoms of schizophrenia, epilepsy, tinnitus, motor stroke, Parkinson´s disease and also neuropatic pain.
It was suggested that TMS could enhance certain cognitive abilities. The research focused on the enhancement of global cognitive functions, attention, executive functions, processing speed, working memory, visual memory, verbal memory and visuospatial activity. However, Donel M. Martin and his colleagues, who conducted meta-analysis of this cognitive enhancement, claim that only working memory appears to enhance significantly after TMS treatment. Other cognitive abilities did not demonstrate significant differences after TMS. In addition, the enhancement of working memory did not appeared among healthy participant but among patients with schizophrenia. The researchers also point out that the number of studies, which shows the improvement of working memory after TMS was considerably small.[19]
Depression
Depression is an umbrella term for common mood disorders. They affected severely daily activities of the patients.[20] In 1950 were developed anti-depressant drugs, but the disorder of approximately 15-30% patients cannot be controlled by medications. Therefore, researchers were interested in new forms of treatment, TMS is one of them.[21]
TMS effect on drug-resistant depression was proved for the first time by Pascual-Leone and his colleagues in 1996. Each patient was stimulated in one of three centres: verex, right dorsolateral prefrontal cortex or left dorsolateral prefrontal cortex (DLPFC). The researchers used the high frequency (10Hz).[22][23] In the "Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation", Jean-Pascal Lefaucheur and his colleagues claim that there are two ways of TMS, which are prevalent in the current research: the low frequency stimulation of right DLPFC and the high frequency stimulation of left DLPFC. While the former stimulation leads to inhibition of areas which are hyperactive during depression, the latter stimulation increase the activity in the areas which are hypoactive during depression. The combination of both methods could be also used. They also point out that TMS treatment of depression is the most efficient if it is applied in young patients, whose disorder is in an acute state and who possess a limited level of treatment resistance.[24]
Schizophrenia
Schizophrenia is a mental disorder characterized by abnormal social behavior and failure to understand the reality. Common symptoms include delusions, unclear or confused thinking, hallucinations. It also includes negative symptoms such as reduced social engagement and emotional expression, and a lack of motivation. Schizophrenia is treated with antipsychotics in general.
Pharmacoresistant symptoms like chronic auditory hallucinations and negative symptoms could be treated with rTMS with possible (propable) effect. [25] Auditory hallucinations are treated with repeated sessions of low frequency rTMS, where the magnetic coil is focused on left temporoparietal cortex. This leads to the decrease of increased cortical excitability at this area. Negative sypmtoms of schizophrenia are treated by high frequency rTMS, where the magnetic coil is focused on the left DLPFC. This leads to the activation of inhibited DLPFC.
Tinnitus
Tinnitus is a symptom defined by hearing of sound when no external sound is present. While often described as a ringing, it may also sound like a clicking, hiss or roaring. Tinnitus can result from a number of underlying causes such as noise-induced hearing loss, ear infections, disease of the heart or blood vessels, Ménière's disease, brain tumors, emotional stress, exposure to certain medications, a previous head injury, and earwax. It can be treated by progressive tinnitus management, tinnitus retraining therapy or psychotherapy such as cognitive behavioral therapy (CBT), acceptance and commitment therapy (ACT) and relaxation techniques. Pharmacological treatment includes predominantly the use of antidepressants, benzodiazepines and anticonvulsants.
There is evidenced a possible effect of single sessions theta burst stimulation (TBS) or low frequency rTMS of the auditory cortex in the treatment of tinnitus.[4] [26]
Neuropatic pain
Neuropathic pain is pain caused by damage or disease affecting the somatosensory nervous system and may be associated with abnormal sensations (dysesthesia, allodynia). It may have continuous and/or episodic (paroxysmal) components. Neuropathic pain can be treated by anticonvulsants (such as pregabalin, gabapentin, carbamazepine and oxcarbazepine), antidepressants (dual serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, or bupropion).
rTMS is used for the treatment of pharmacoresistant neuropatic pain. The magnetic coil is targeted on M1 motor cortex contralateral to pain side and high frequency of rTMS (10 Hz, 20 Hz, or theta burst stimulation 50 Hz) is used in the repeated sessions. There is confirmed the definite analgetic effect of high frequency rTMS in the treatment of neuropatic pain. [4]
Migraine
Migraine is a neurological disorder which is one of the most common causes of disability.[27] The patients suffering from migraine report symptoms as headache, nausea, vomiting, sound and light sensitivity. The migraine could be with or without aura. Certain patients have also migraine without headache but with aura. There is no treatment which could cure migraine, but several treatments handle with its symptoms. There are drugs as painkillers, triptans or anti-emetics which could deal with certain symptoms of migraine.[28]
For the treatment of migraine's symptoms, Cerena was approved by FDA in 2013 as the first device. The stimulation is based on single-pulse TMS (sTMS).[29] However, the results show that TMS is more efficient in the case of patients suffering from the migraine with aura. The migraine with aura is accompanied with cortical spreading depression, which give a rise of migraine pain. TMS could disturb these condition, which leads to the reduction of headache. However, the migraine without aura could be also positively affected by TMS. The results are not as significant as in the case of migraine with aura.[5] There are several possible TMS, which could affect migraine. The treatment provided by devices manufactured by eNeura (Cerena, SpringTMS) consists in sTMS. It is stimulated the area below the occipital bone.[30] However, evidence based guidelines on the therapeutic use of rTMS do not recommend rTMS for the treatment of migraine.[4] for the disputed effect of the method in this case.
rTMS is also currently examined on the treatment of other diseases and disorders such as epilepsy and Parkinson´s disease[4], Alzheimer's disease[31], obsessive-compulsive disorder,[32] autism spectrum disorder[33], or post-stroke recovery.[34]
Ethical & Health Issues
TMS does not have a community of enthusiasts as large as the tDCS does. However, the approvals from the FDA mean that these devices are soon to be considered safe for treatment of more patients and that means the technology will move closer to the regular consumer. Excluding ethical issues perhaps more relevant to the clinical use of the TMS, i.e. issues such as the need of informed consent of the patient, pre-treatment observations, and long-term effects research, there are other potential ethical concerns TMS, or rather any other brain stimulation technique may pose.[13][35]
This technology can introduce unwanted, although short-lasting, mood changes as depression or suicidality.[36] It can be used to alter cognition, namely it could impair certain cognitive abilities.[35] It could also lead to unexpected side-effects or, in the worst cases, to serious health issues (discussed below). Because brain stimulation replaces the effect of pharmaceuticals, it can be abused as a form cheating or doping while being undetectable by chemical tests (see tDCS entry for more).
While non-invasive, transcranial magnetic stimulation is not without unwanted side-effects. The ability to easily influence the human brain and subsequently the entire body and thus health of the patients raises great concerns about safety and procedure protocol of the technique. The adverse effects of TMS can include but are not limited to inducing seizure, headache, syncope, phosphenes, dysphoria, uncontrollable laughter, speech arrest, scotoma, or skin burns from electrodes (if used).[37]
Other health concerns are linked with the procedure itself. The coil produces a high-intensity 'click' sound when a TMS is turned on, with enough sound pressure to damage hearing. This could be, however, avoided with the use of plugs. In addition, if the stimulation could be ineffective, if the design of the stimulation or the patient is not appropriate. The device could also interfere negatively with other devices and therapies.[36]
Public & Media Impact and Presentation
Since TMS was approved for the treatment of depression, its review appears in certain newspapers. The journalists listed pros and cons of the treatment. They reported some life-changing experiences, as the one of Martha Rhodes:
After four weeks, “I woke up and something was different,” said Mrs. Rhodes, who wrote a book, “3,000 Pulses Later” describing the treatment. “I felt lighter. I didn’t wake up in the morning and wish I were dead.”[38]
It is also considered to be safe treatment with minimum side effects:
I can reassure people as to the safety of TMS, in that I've experienced it several times myself by volunteering for studies at the Cardiff University Brain Research Imaging Centre. I only ever had one experience that alarmed me. During one study, I was having my motor cortex activated, which caused my arm to flail involuntarily (it sounds worrying, but it's essentially a hi-tech version of a doctor testing your reflexes in your knee with a mallet). This experience didn't hurt, and as a neuroscience enthusiast I found the experience cool rather than worrying.[39]
The journalists also point out that TMS has certain limits, namely it is considerably expensive and time consuming:
It sounds all good, but there is a downside. Treatments are very time consuming: five days a week and almost an hour a day for six weeks. And the cost for a full series will be in the neighborhood of $10,000. Insurance is gradually starting to pay, depending on the state and for some medication must be continued, both during the treatments and after.[40]
In addition, although there are certain clues for which patients TMS is appropriate, the overall characteristics of appropriate candidate of TMS is unknown:
“While it’s fairly clear that TMS is effective in some percentage of patients with major depressive disorder, it’s still not very easy to know in advance who those patients are,” said Dr. Steven J. Zalcman, the head of the clinical neuroscience research branch of the National Institute of Mental Health.[38]
Finally, it is not as efficient as electroconvulsive therapy (ECT), even though, it has less side-effects:
My conversation with him showed that although TMS is a viable procedure, it fails to come close to the effectiveness rates of ECT. There are significantly fewer side effects with TMS, however, so TMS might be a good alternative for some.[41]
TMS is also discussed in public. There is a sub-reddit, focused on rTMS therapy. In comparison with a sub-reddit focused on tDCS who is more linked with cognitive enhancement' enthusiasts and Do-It-Yourself community, the debaters at rTMS sub-reddit recruit mainly from patients considering the treatment of those who were already treated with TMS. There are many positive experiences as:
For me rTMS has worked remarkably well, While it has not completely alleviated my symptoms, it has allowed me to go from 600mg of Ketamine per week to under 150mg of Ketamine per week. In fact I think I am down to 1 dose of ketamine every 10 days or so. Considering I was non functional before the ketamine this is a dramatic improvement.[42]
There are also patients, who underwent TMS, but the treatment was ineffective:
...Either way, yesterday I wrapped up 36 sessions of rTMS treatment on a Neurostar system. Clinical data says this has long lasting effects to treat depression for about 1/3rd of people, has shorter lasting effects for 1/3rd of people, and doesn't effect the rest. Where I had it done, they told me something along the lines of "We've treated 64 patients, very few have left without a positive effect". I guess I threw off their winning streak.[43]
Another debater at Reddit was afraid that the treatment could affected his personality:
I have a fear (possibly irrational) that I will start to lose my identity which I've held as a "depressed" person. I've been in therapy and tried many meds the last 15 years, since I was 18. Meds wouldn't work because I felt like they changed me too much. As a person I didnt relate to the person I would become on meds. [44]
Debra Drake, who wrote a blog about her struggle with depression, stress certain drawbacks of her TMS sessions. She argues that the treatment was not entirely efficient and the procedure was expensive and painful:
Overall, I'd give my TMS experience a B-. I think it helped my medications work better, and helped me get into a little routine which I wasn't used to before. And even though I'm tired as heck all the time, I'm finally able to fight against sleeping all day. I also get really antsy now when I'm not doing little projects. I gave it a B- because of it's extremely high price, and the procedure itself was very emotional for me because of the pain I had to endure. Not. Fun. One. Bit.[45]
It is considerably more expensive to build TMS device than to build tDCS device. However, there appeared at Reddit a manufacturer who proposed an expedition of Do-It-Yourself TMS device.[46] We have not been able to find any review of this device yet.
Public Policy
rTMS was recognised as one of the options of a treatment of depression by American Psychiatric Association (APA), the Canadian Network for Mood and Anxiety Treatments (CANMAT), and the World Federation of Societies of Biological Psychiatry (WFSBP).[24] It was approved by FDA in 2008.[17]
sTMS was evaluated as cost-effective in the treatment of migraine headache with aura. Cerena device was listed among class II devices.[18]
Related Technologies, Projects, or Scientific Research
There are alternative therapies for the treatment of disorders for which TMS was approved. Depression could be treated with antidepressant medication, psychotherapy, talk therapy, or electroconvulsive therapy (ECT).[47] Sooma tDCS™ which provide transcranial direct-current stimulation (tDCS) therapy was also approved for the treatment of depression in Europe.[48] The depression was also experimentally treated with Deep brain stimulation (DBS).[49]
The migraine could be treated with medication and healthy life-style.[50] There are also alternative treatments as acupuncture, neurofeedback, massage therapy or cognitive behavioural therapy,[51] and vagus nerve stimulation.[52]
References
- ↑ 1.0 1.1 ELDAIEF, Mark C., PRESS, Daniel Z. and PASCUAL-LEONE, Alvaro, Transcranial magnetic stimulation in neurology A review of established and prospective applications. Neurology: Clinical Practice. 2013. 3(6), 519–526. Doi: 10.1212/01.CPJ.0000436213.11132.8e Available online at: https://www.ncbi.nlm.nih.gov/pubmed/24353923
- ↑ 2.0 2.1 HALLETT, Mark. Transcranial Magnetic Stimulation: A Primer. Neuron. 2007. 55(2), 187–199. Doi: 10.1016/j.neuron.2007.06.026. (Retrieved 3rd May, 2017).
- ↑ SLOTEMA, C.W., BLOM, J.D., HOEK, H.W., SOMMER, I.E. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. The Journal Clinical Psychiatry, 2010, 71(7), 873–884. Doi: 10.4088/JCP.08m04872gre. Available online at: http://www.sydneytms.com.au/wp-content/uploads/2015/03/TMS_Meta_Analysis.pdf (Retrieved 5th May, 2017).
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Lefaucheur,J.P., Andre-Obadia, n., Antal, a., Ayache, S.S., Baeken, c., Benninger, D.H. et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clinical Neurophysiology, 2014, 125(11), 2150–2206. Available online at: http://www.sciencedirect.com/science/article/pii/S138824571400296X (Retrieved 18th May, 2017).
- ↑ 5.0 5.1 LIPTON, Richard B. et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. The Lancet: Neurology. 2010, Apr. 9(4), 373–380 Doi: 10.1016/S1474-4422(10)70054-5 Available online at: http://www.sciencedirect.com/science/article/pii/S1474442210700545 (Retrieved 2nd May, 2017).
- ↑ Magstim [online]. Available online at: https://www.magstim.com/article/13/magstim-announces-fda-510k-clearance-of-rtms-repetitive-transcranial-magnetic-stimulation-therapy-system (Retrieved 3rd May, 2017).
- ↑ Magventure [online]. Available online at: http://www.magventure.com/en-gb/About/News-and-Events/News/ID/1057/MagVenture-receives-FDA-clearance-for-TMS-depression-treatment-system (Retrieved 3rd May, 2017).
- ↑ Neurostar TMS [online]. Available online at: https://neurostar.com/ (Retrieved 3rd May, 2017).
- ↑ Brainsway. About Brainsway. Brainsway [online]. Available online at: http://www.brainsway.com/about-brainsway (Retrieved 3rd May, 2017).
- ↑ RIDDING, Michael C. and ROTHWELL, John C. Is there a future for therapeutic use of transcranial magnetic stimulation? Nature reviews. Neuroscience. 2007. 8(7), 559–567. Doi: 10.1038/nrn2169 Available online at: http://www.nature.com/nrn/journal/v8/n7/full/nrn2169.html (Retrieved 3th May, 2017).
- ↑ WAGNER, Timothy, VALERO-CABRE, Antoni and PASCUAL-LEONE, Alvaro. Noninvasive human brain stimulation. Annual Review of Biomedical Engineering [online]. 2007. 9(1), 527–565. Doi: 10.1146/annurev.bioeng.9.061206.133100. Available online at: http://dx.doi.org/10.1146/annurev.bioeng.9.061206.133100\nfiles/392/Wagner et al. - 2007 - Noninvasive Human Brain Stimulation.pdf (Retrieved 3rd May, 2017).
- ↑ KOBAYASHI, Masahito and PASCUAL-LEONE, Alvaro. Transcranial magnetic stimulation in neurology. The Lancet: Neurology. 2003. 2(3), 145–156. Doi: 10.1016/S1474-4422(03)00321-1. Available online at: http://www.sciencedirect.com/science/article/pii/S1474442203003211 (Retrieved 3rd May, 2017).
- ↑ 13.0 13.1 13.2 13.3 13.4 HORVATH, J. C., et al. Transcranial magnetic stimulation: a historical evaluation and future prognosis of therapeutically relevant ethical concerns. Journal of medical ethics. 2011, 37(3), 137–43. Doi: 10.1136/jme.2010.039966 Available online at: https://www.ncbi.nlm.nih.gov/pubmed/21106996 (Retrieved 26th April, 2017).
- ↑ 14.0 14.1 PASCUAL‐LEONE, Alvaro; GATES, John R.; DHUNA, Anil. Induction of speech arrest and counting errors with rapid‐rate transcranial magnetic stimulation. Neurology. 1991, 41(5), 697-702.
- ↑ 15.0 15.1 KOLBINGER, H. M. et al. Transcranial Magnetic Stimulation (TMS) in the Treatment of Major Depression - a Pilot-Study. Human Psychopharmacology-Clinical and Experimental [online]. 1995. 10(4), 305–310. Doi: 10.1002/Hup.470100408 Available online at: http://onlinelibrary.wiley.com/doi/10.1002/hup.470100408/abstract (Retrieved 26th April, 2017).
- ↑ 16.0 16.1 WASSERMANN, E. M. and LISANBY, S. H. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology [online]. 2001, 112(8), 1367–77. Doi: 10.1016/S1388-2457(01)00585-5. Available online at: http://www.sciencedirect.com/science/article/pii/S1388245701005855 (Retrieved 26th April, 2017).
- ↑ 17.0 17.1 17.2 O’REARDON, John P. et al. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biological Psychiatry. 2007. 62(11), 1208–1216. Doi: 10.1016/j.biopsych.2007.01.018.
- ↑ 18.0 18.1 18.2 FOY, Jonette. Cerena Transcranial Magnetic Stimulator: Evaluation of Automatic Class III Designation – De Novo Request. U.S. Food and Drug Administration [online]. 2013, Dec 13. Available online at: https://www.accessdata.fda.gov/cdrh_docs/pdf13/K130556.pdf (Retrieved 2nd May, 2017).
- ↑ MARTIN, Donel M. et al. Does Therapeutic Repetitive Transcranial Magnetic Stimulation Cause Cognitive Enhancing Effects in Patients with Neuropsychiatric Conditions? A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Neuropsychology Review. 2016, Sep. 26(3), 295–309 Doi: 10.1007/s11065-016-9325-1 Available online at: http://link.springer.com/article/10.1007/s11065-016-9325-1 (Retrieved 3rd May, 2017).
- ↑ The National Institute of Mental Health. Depression. The National Institute of Mental Health [online]. Available online at: https://www.nimh.nih.gov/health/topics/depression/index.shtml (Retrieved 2nd May, 2017).
- ↑ HERWIG, U. et al. Antidepressant effects of augmentative transcranial magnetic stimulation. British Journal of Psychiatry. 2007, 191(5), 441-448. Doi: 10.1192/bjp.bp.106.034371 Available online at: http://bjp.rcpsych.org/content/bjprcpsych/191/5/441.full.pdf (Retrieved 2nd May, 2017).
- ↑ PASCUAL-LEONE, Alvaro et al. Rapid-rate transcranial magnetic stimulation of left DLPFC in drug-resistant depression. The Lancet [online]. 1996, Jul 27. 348(9022), 233–237. Doi: https://doi.org/10.1016/S0140-6736(96)01219-6 Available online at: http://www.sciencedirect.com/science/article/pii/S0140673696012196 (Retrieved 2nd May, 2017).
- ↑ ZIEMANN, Ulf. Thirty years of transcranial magnetic stimulation: where do we stand? Experimental Brain Research. 2017, 235(4), 973–984. Doi: 10.1007/s00221-016-4865-4 Available online at: http://link.springer.com/article/10.1007%2Fs00221-016-4865-4 (Retrieved 2nd May, 2017).
- ↑ 24.0 24.1 LEFAUCHEUR, Jean-Pascal et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clinical Neurophysiology. 2014, Nov. 125(11), 2150–2206. Doi: 10.1016/j.clinph.2014.05.021 Available online at: http://www.sciencedirect.com/science/article/pii/S138824571400296X (Retrieved 2nd May, 2017).
- ↑ DOUGALL N. et al. Transcranial magnetic stimulation (TMS) for schizophrenia. Cochrane Database of Systematic Reviews. 2015, 8. Art. No.: CD006081. DOI: 10.1002/14651858 Available online at: http://www.cochrane.org/CD006081/SCHIZ_transcranial-magnetic-stimulation-tms-treatment-schizophrenia (Retrieved 3rd May, 2017).
- ↑ LEFAUCHEUR, Jean-Pascal et al. Navigated rTMS for the treatment of tinnitus: A pilot study with assessment by fMRI and AEPs. Neurophysiologie Clinique/Clinical Neurophysiology. 2012, Apr. 42(3), 95–109. Doi: 10.1016/j.neucli.2011.12.001 Available online at: http://www.sciencedirect.com/science/article/pii/S0987705311002358 (Retrieved 3rd May, 2017).
- ↑ ANDREOU, Anna P. et al. Transcranial magnetic stimulation and potential cortical and trigeminothalamic mechanisms in migraine. Brain. 2016, May 30. 139(7), 2002-2014. Doi: https://doi.org/10.1093/brain/aww118 Available online at: https://academic.oup.com/brain/article-lookup/doi/10.1093/brain/aww118 (Retrieved 2nd May, 2017).
- ↑ NHS Choices. Migraine. NHS Choices [online]. Available online at: http://www.nhs.uk/Conditions/Migraine/Pages/Introduction.aspx (Retrieved 2nd May, 2017).
- ↑ eNeura, Inc. eNeura, Inc. Receives FDA Clearance for SpringTMS® Migraine Treatment Device. PR Newswire [online]. 2014, May 23. Available online at: http://www.prnewswire.com/news-releases/eneura-inc-receives-fda-clearance-for-springtms-migraine-treatment-device-260398971.html (Retrieved 2nd May, 2017).
- ↑ BARKER, Anthony T. and SHIELDS, Kevin. Transcranial Magnetic Stimulation: Basic Principles and Clinical Applications in Migraine. Headache. 2016, Dec 28. 57(3), 517-524. DOI: 10.1111/head.13002 Available online at: https://www.ncbi.nlm.nih.gov/labs/articles/28028801/ (Retrieved 2nd May, 2017).
- ↑ RABEY, Jose M. et al. Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: a randomized, double-blind study. Journal of Neural Transmission. 2013, May. 120(5), 813–819. Doi: 10.1007/s00702-012-0902-z Available online at: http://link.springer.com/article/10.1007/s00702-012-0902-z/fulltext.html (Retrieved 3rd May, 2017).
- ↑ ZHOU, Dong-Dong et al. An updated meta-analysis: Short-term therapeutic effects of repeated transcranial magnetic stimulation in treating obsessive-compulsive disorder. Journal of Affective Disorders, 2017, Jun. 215, 187–196. Doi: 10.1016/j.jad.2017.03.033 Available online at: http://www.sciencedirect.com/science/article/pii/S0165032716317967 (Retrieved 3rd May, 2017).
- ↑ OBERMAN, Lindsay M. et al. Transcranial magnetic stimulation in autism spectrum disorder: Challenges, promise, and roadmap for future research. Autism Research. 2016, Feb. 9(2), 184–203 Doi: 10.1002/aur.1567 Available online at: http://onlinelibrary.wiley.com/doi/10.1002/aur.1567/abstract (Retrieved 3rd May, 2017).
- ↑ HUMMEL F. C. et al. Controversy: noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimulation. 2008, Oct. 1(4), 370–82. Doi: 10.1016/j.brs.2008.09.003 Available online at: http://www.sciencedirect.com/science/article/pii/S1935861X08003367 (Retrieved 3rd May, 2017).
- ↑ 35.0 35.1 ROSSI, Simone et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology [online]. 2009. 120(12), 2008–2039. Doi: 10.1016/j.clinph.2009.08.016. Available online at: http://dx.doi.org/10.1016/j.clinph.2009.08.016 (Retrieved 3rd May, 2017).
- ↑ 36.0 36.1 U.S. Food and Drug Administration. Guidance for Industry and FDA Staff - Class II Special Controls Guidance Document: Repetitive Transcranial Magnetic Stimulation (rTMS) Systems. U.S. Food and Drug Administration [online]. Available online at: https://www.fda.gov/RegulatoryInformation/Guidances/ucm265269.htm (Retrieved 3rd May, 2017).
- ↑ WASSERMANN, Eric M., 1998, Risk and safety of repetitive transcranial magnetic stimulation. Electroencephalography and Clinical Neurophysiology [online]. 108(1), 1–16. Doi: 10.1016/S0168-5597(97)00096-8 Available online at: http://www.ncbi.nlm.nih.gov/pubmed/9474057 (Retrieved 3rd May, 2017).
- ↑ 38.0 38.1 RABIN, Roni Caryn. New Approach to Depression. Well Blogs New York Times [online]. 2013, Jul 1. Available online at: https://well.blogs.nytimes.com/2013/07/01/new-approach-to-depression/?_r=0 (Retrieved 4th May, 2017).
- ↑ BURNETT, Dean. Brain-controlling magnets: how do they work? The Guardian [online]. 2014, May 17. Available online at: https://www.theguardian.com/science/brain-flapping/2013/may/17/brain-controlling-magnets-how-do-they-work (Retrieved 4th May, 2017).
- ↑ ARCHER, Dale. Transcranial Magnetic Stimulation (TMS) Treats Depression. PsychologyToday [online]. 2014, Jan 28. Available online at: https://www.psychologytoday.com/blog/reading-between-the-headlines/201401/transcranial-magnetic-stimulation-tms-treats-depression (Retrieved 4th May, 2017).
- ↑ HERSH, Julie K. TMS or ECT? A Mental Health Consumer Weighs the Options: When should a patient use ECT or TMS? PsychologyToday [online]. 2013, Jun 27. Available online at: https://www.psychologytoday.com/blog/struck-living/201306/tms-or-ect-mental-health-consumer-weighs-the-options (Retrieved 4th May, 2017).
- ↑ BRUMBELOW, R. K. R.K. Brumbelow's experience with rTMS. Reddit [online]. 2016, Sep 14. Available online at: https://www.reddit.com/r/rtms/comments/4jrj3a/rk_brumbelows_experience_with_rtms/ (Retrieved 4th May, 2017).
- ↑ THIRDPROJECTJUNO. TMS didn't work for me. If it works for 66% of people, that means your odds are now very slightly higher. Reddit [online]. 2016, Nov 23. Available online at: https://www.reddit.com/r/rtms/comments/5ejkyo/tms_didnt_work_for_me_if_it_works_for_66_of/ (Retrieved 4th May, 2017).
- ↑ SOLOMONROOTS. Identity issues. Reddit [online]. 2017, Apr 21. Available online at: https://www.reddit.com/r/rtms/comments/66sh2m/identity_issues/ (Retrieved 4th May, 2017).
- ↑ DRAKE, Debra. My TMS Experience. Deb's Journey [online]. 2012, Jun 22. Available online at: http://debsfitnessjourney.blogspot.cz/2012/06/my-tms-experience.html (Retrieved 4th May, 2017).
- ↑ QUICKSILV3RFLASH. Preorder an (affordable) rTMS device to personally own. Reddit [online].2017, Mar 30. Available online at: https://www.reddit.com/r/rtms/comments/62b8r7/preorder_an_affordable_rtms_device_to_personally/ (Retrieved 4th May, 2017).
- ↑ WebMD. Major Depression (Clinical Depression). WebMD [online]. Available online at: http://www.webmd.com/depression/guide/major-depression#2-8 (Retrieved 4th May, 2017).
- ↑ Somamedical. Sooma tDCS. Somamedical [online]. Available online at: http://soomamedical.com/wp-content/uploads/2016/11/C2015-014-A05-White-Paper-EN.pdf (Retrieved 4th May, 2017).
- ↑ MAYBERG, Helen S. et al. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron. 2005, 45(5), 651–660. Available online at: http://www.sciencedirect.com/science/article/pii/S089662730500156X (Retrieved 5th May, 2017).
- ↑ NHS Choices. Migraine - Prevention. NHS Choices [online]. Available online at: http://www.nhs.uk/Conditions/Migraine/Pages/Prevention.aspx (Retrieved 5th May, 2017).
- ↑ Mayo Foundation for Medical Education and Research. Migraine: Diagnosis and treatment. Mayo Foundation for Medical Education and Research [online]. Available online at: http://www.mayoclinic.org/diseases-conditions/migraine-headache/diagnosis-treatment/dxc-20202471 (Retrieved 5th May, 2017).
- ↑ National Institute for Health and Care Excellence. Transcutaneous stimulation of the cervical branch of the vagus nerve for cluster headache and migraine. National Institute for Health and Care Excellence [online]. 2016, Mar. Available online at: https://www.nice.org.uk/guidance/IPG552/ifp/chapter/what-has-nice-said (Retrieved 5th May, 2017).