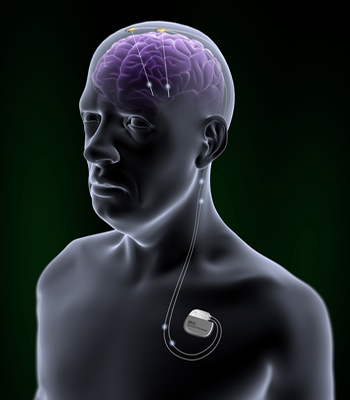
Brio™ Rechargeable IPG
| Brio™ Rechargeable IPG | |
|---|---|

|
|
| Category | Deep brain stimulation |
| Developer | St. Jude Medical, Inc. [1] |
| Announced | 2008 [2] |
| Released | Developers:
Consumers: 2008 [1] |
| Price | 19 150 USD [3] |
| Max output | 12.512.5 T 12.5 mA 0.0125 A mA[2] |
| Session duration | 0.050.05 s 8.333334e-4 minute s each pulse, the current is delivered constantly[2] |
| Scalp location | subthalamic nucleus, globus pallidus internus, ventral intermediate nucleus [4][5][6] |
| Weight | 29 g [1] |
| Controls |
Brio™ Patient Programmer, magnet [6] |
| Data available | |
| Risk factor | |
| Medical prescription | yes |
| https://www.sjmglobal.com/en-int/professionals/featured-products/neuromodulation/deep-brain-stimulation/implantable-pulse-generators/brio-ipg | |
Brio™ Rechargeable IPG (Implantable Pulse Generator) provides deep brain stimulation therapy for patients suffering by Parkinson disease and essential tremor. It does not cure the diseases but it could help to reduce their symptoms. It is used primarily in the cases when the medication of the disease is not successful.[7]
The device was developed by St. Jude Medical, Inc. the company which focus on the medical devices and is settled in St. Paul, Minnesota.[8]
Contents
Main characteristics
Brio™ Rechargeable IPG was developed as a medical device which could provide treatment through deep brain stimulation. It is a standalone device and could be received only on prescription. It has to be implanted only on special clinics and the quality of surgery has a huge impact on the results of the treatment.[6] The device was approved by as a treatment of symptoms of Parkinson disease, essential tremor,[9] and Dystonia.[10]
The device consists of:
- Implantable pulse generator (IPG)
- Extensions
- Leads
- Patient programmer
- Patient magnet
- Charging system
The leads are implanted into the brain in accordance with the purposed treatment. The IPG is implanted on the upper part of the chest and could be controlled by patent programmer. Magnet could turn on or of the IPG.[6]
The IPG provides a long term brain stimulation. It contains a rechargeable battery. The device could be charged via antenna.[11] Brio™ Rechargeable IPG is a system which operate open-loop. It means that the IPG delivers continuous stimulation to patient's brain.[12] In addition, Brio™ Rechargeable IPG provides a constant-current stimulation, which means that the IPG deliver constant current to the brain. It is contrasted with voltage-controlled stimulation, in which the amount of affected tissue may vary.[5]
Purpose
The main purpose of the device is to treat the symptoms of certain incurable diseases and improve the patients' life in this way.
Company & People
The device was originally manufactured by St. Jude Medical, Inc, the company from St. Paul, Minnesota.[9] This company was however acquired by Abbott Laboratories in 2017.[13] Abbott Laboratories seats in Abbott Park, Illinois.[14]
- Miles D. White - Chairman and Chief Executive Officer of Abbott Laboratories
- Hubert L. Allen - Executive Vice President, General Counsel and Secretary of Abbott Laboratories[15]
- Michael T. Rousseau - President, Cardiovascular and Neuromodulation of Abbott Laboratories, former CEO of St. Jude Medical, Inc.[16]
Important Dates
- 1976 - the company St. Jude Medical, Inc. was founded.[17]
- 2009 - Brio™ Rechargeable IPG received CE (Conformité Européenne) mark of approval for treatment of Parkinson disease.[9]
- 2010 - Brio™ Rechargeable IPG was approved by the Australian Therapeutic Goods Administration (TGA).[8]
- 2013 - Brio™ Rechargeable IPG received CE (Conformité Européenne) mark of approval for treatment of Dystonia symptoms.[10]
- 2015 - Brio™ Rechargeable IPG was approved by FDA.[7]
- 2017 - St. Jude Medical, Inc. was acquired by Abbott Laboratories.[13]
Enhancement/Therapy/Treatment
Brio™ Rechargeable IPG is a medical device, which was developed as a treatment of symptoms of Parkinson's disease, Essential Tremor[9] and Dystonia[10]. It is not meant for cognitive enhancement, but the possibility of certain cognitive enhancement has also not been excluded yet.
Parkinson's disease
Parkinson's disease is neurodegenerative disease. It is caused by the loss of brain cells which produce dopamine.[18] Its symptoms could be resting tremor, muscular rigidity, bradykinesia, and postural instability.[19] The symptoms appear gradually and the progression of the disease differs among patients. Parkinson's disease affects primarily the population over 60. The disease in incurable at present.[18]
Brio™ Rechargeable IPG is one of deep brain stimulation devices which could treat the symptoms of Parkinson's disease. The suggested treatment consists in bilateral stimulation of the subthalamic nucleus. This treatment focuses on the patients which disease cannot be controlled by medications.[6]
Essential Tremor
Essential tremor is a movement disease, when one or more parts of patient's body move unintentionally. The movement is rhythmic and the affected parts oscillate.[20] It could be non-progressive but it is usually progressive. The disease is widespread primarily among population over 60.[12]
There has not been developed the medication specially for the treatment of essential tremor. Certain drugs are used off-label to the treatment, but approximately 50% of patients cannot tolerated any medication or the symptoms persist even after the medication. These patients could be treated by lesional therapies or brain stimulation,[12] if the illness possess a significant functional disability.[6]
Brio™ Rechargeable IPG treat the essential tremor by the unilateral or bilateral stimulation of the ventral intermediate nucleus, which is a part of thalamus.[6]
Dystonia
Dystonia is a movement disorder, which consists in an involuntary movement as the contraction of muscles, repetitive movements, spasms, etc. The movement is often painful. Dystonia could appear in any age but the many patients are diagnosed in their childhood or early adulthood. There are more types of dystonia. The primarily or idiopathic dystonia is not caused by the injury or disease and might be inherited.[21] In contrast, the secondary dystonia could be caused by trauma, drugs exposure, tumour or other disease.[10]
There is no treatment which could be effective in the treatment of all patients with dystonia.[21] When the medication is not successful, patients could be treated also with deep brain stimulation. In the case of dystonia, the stimulation of Globus pallidus or the ventral intermedius nucleus seems to be more efficient.[10]
Ethical & Health Issues
Although Brio™ Rechargeable IPG could bring relief patients suffering Parkinson's disease, essential tremor and dystonia, there are many health issues linked with the device. They are identified in "Brio™ Patient Programmer: User's Guide"[6] or the report provided by FDA.[2]
The first important issue is the selection of suitable patients. The patient should be able to handle with the device and the programmer. Additionally, the doctor should test the patient carefully, in order to decide if this treatment is suitable for the disease the patient is suffering. Certain therapies could also negatively interfere with the device. These are therapies as electroshock therapy, transcranial magnetic stimulation, diathermy, magnetic resonance, external defibrillators, and therapeutic radiation. The device could also negatively interfere with other devices as implanted peacemaker, microwave, mobile phones, theft detectors, metal screening devices, appliances containing magnet etc. The device should not be also implanted to pregnant or nursing women.[6]
Several issues are linked with the surgery. The complications as haemorrhage or cerebrospinal fluid leakage could appear during surgery. The main complication after surgery is infection. After the surgery, patients also reported side-effects as dysarthria, fatigue, postoperative pain and discomfort, paraesthesias, and oedema.[4] Patients could also struggle with the depression and suicidal intentions after surgery.[6]
The everyday use of the device could also arise certain issues. Patients are warned not to use extensive stimulation and charge density, since the high amplitudes and wide pulses could damage their tissue. The low frequency, in contrast, could cause tremor. Since the device includes rechargeable battery, the device has to charge regularly. The charging could be painful or uncomfortable for patients, because the device heated during the charging.[6] However, the rechargeable battery significantly reduces the number of surgeries and the use of a rechargeable device is cheaper than the use of a non-rechargeable device. Therefore, the rechargeable devices as Brio™ Rechargeable IPG is favoured.[22] Patients could also encounter issues linked with the stimulation itself as: speech or language impairment including, aphasia, dysphagia, dysarthria, and hypophonia, supranuclear gaze palsy, hypersexuality or increased libido, or worsening existing medical conditions.[6]
It was reported a case of a series of Brio™ Rechargeable IPG, where the IPG ceased delivering the stimulation. It was caused by the faulty battery component, which prevent charging of the device and communication with the programmer. St. Jude Medical, Inc. identified the problem and sent "Field Safety Notice" to the doctors, with the serial numbers of the devices which might be affected by this failure. St. Jude Medical, Inc. offered a replacement of the devices affected by the failure which had not been implanted and those which had been implanted and failed to deliver the stimulation. In other cases, they recommended a careful monitoring of the patients, which have implanted devices which could contain faulty battery.[23] There were also reported 11 cases in which the leakage of body fluids interrupted therapy. St. Jude Medical, Inc. pulled off all devices which were not implanted and warn the doctors as in the previous case. They recommended the use of Libra™.[24]
Public & Media Impact and Presentation
"There are no cures for Parkinson's disease or essential tremor, but finding better ways to manage symptoms is essential for patients," says Dr. William Maisel of the FDA. "This new device adds to the array of treatment options to help people living with Parkinson's and essential tremor enjoy better, more productive lives."[25]
It might not be covered by insurance - http://www.mdedge.com/neurologyreviews/article/104119/movement-disorders/what-future-dbs-movement-disorders
Market - http://medcitynews.com/2011/01/st-jude-medical-to-sell-deep-brain-stimulation-device-in-australia/
Small device - http://www.medgadget.com/2015/06/st-judes-brio-neurostimulator-for-parkinsons-essential-tremor-fda-approved.html
the advantage of DBS - http://practicalneurology.com/2015/06/new-option-now-available-for-dbs-for-pd-tremor/
MOre available devices - https://www.michaeljfox.org/foundation/news-detail.php?fda-approves-new-deep-brain-stimulation-device
Public Policy
Related Technologies, Projects or Scientific Research
St. Jude Medical, Inc. developed also two other deep brain stimulation devices Libra™ and Infinity™ DBS IPG.
Brio™ Rechargeable IPG was examined in several papers:
References
- ↑ 1.0 1.1 1.2 St. Jude Medical. St. Jude Medical Receives CE Mark Approval for World’s Smallest, Longest-Lasting Rechargeable Deep Brain Stimulator for Parkinson’s Disease. Newswise, Inc [online]. 2009, Sep 9. Available online at: http://www.newswise.com/articles/st-jude-medical-receives-ce-mark-approval-for-world-s-smallest-longest-lasting-rechargeable-deep-brain-stimulator-for-parkinson-s-disease-first-patient-implanted-with-brio-neurostimulator-at-university-of-cologne-germany32 (Retrieved 6th April, 2017).
- ↑ 2.0 2.1 2.2 2.3 U.S. Food and Drug Administration [online]. Summary of Safety and Effectiveness Data. U.S. Food and Drug Administration [online]. Available online at: https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140009b.pdf (Retrieved 11th April, 2017).
- ↑ Australian Government. Private Health Insurance (Prostheses) Rules 2015 (No. 1). Federal Register of Legislation [online]. Available online at: https://www.legislation.gov.au/Details/F2015C00649/Html/Volume_2 (Retrieved 6th April, 2017).
- ↑ 4.0 4.1 OKUN, M. et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. The Lancet Neurology, 2012, 11(2), 140-149. Available online at: http://www.sciencedirect.com/science/article/pii/S1474442211703088 (Retrieved 6th April, 2017).
- ↑ 5.0 5.1 PREDA, F. et al. Switching from constant voltage to constant current in deep brain stimulation: a multicenter experience of mixed implants for movement disorders. European Journal of Neurology, 2016, 23(1), 190–195. Doi:10.1111/ene.12835 Available online at: http://onlinelibrary.wiley.com/doi/10.1111/ene.12835/full (Retrieved 6th April, 2017).
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 St. Jude Medical. Brio™ Patient Programmer. U.S. Food and Drug Administration [online]. Available online at: www.accessdata.fda.gov/cdrh_docs/pdf14/P140009c.pdf (Retrieved 6th April, 2017).
- ↑ 7.0 7.1 U.S. Food and Drug Administration. FDA approves brain implant to help reduce Parkinson’s disease and essential tremor symptoms. U.S. Food and Drug Administration [online]. 2015, Jun 12. Available online at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm451152.htm (Retrieved 6th April, 2017).
- ↑ 8.0 8.1 St. Jude Medical. St. Jude Medical's Brio DBS system for treating Parkinson’s disease receives Australian TGA approval. News-Medical.Net [online]. 2010, Mar 23. Available online at: http://www.news-medical.net/news/20100323/St-Jude-Medicals-Brio-DBS-system-for-treating-Parkinsone28099s-disease-receives-Australian-TGA-approval.aspx (Retrieved 6th April, 2017).
- ↑ 9.0 9.1 9.2 9.3 BUSINESS WIRE. St. Jude Medical Receives CE Mark Approval for World’s Smallest, Longest-Lasting Rechargeable Deep Brain Stimulator for Parkinson’s Disease. BUSINESS WIRE [online]. 2009, Sep 9. Available online at: http://www.businesswire.com/news/home/20090909005095/en/St.-Jude-Medical-Receives-CE-Mark-Approval (Retrieved 6th April, 2017).
- ↑ 10.0 10.1 10.2 10.3 10.4 GUILLIOTTI, Kimberly. St Jude Medical’s Deep Brain Stimulation Trifecta: New Treatment Options for Dystonia in EU. MD Buyline [online]. 2013, Apr 23. Available online at: http://www.mdbuyline.com/st-jude-medicals-deep-brain-stimulation-trifecta-new-treatment-options-for-dystonia-in-eu/ (Retrieved 6th April, 2017).
- ↑ St. Jude Medical, Inc. Brio™ Rechargeable IPG: Avdanced Technology. St. Jude Medical, Inc. [online]. Available online at: https://www.sjmglobal.com/en-int/professionals/featured-products/neuromodulation/deep-brain-stimulation/implantable-pulse-generators/brio-ipg?halert=show&clset=92f57278-460e-4300-b7fe-89e52a04194f%3acadddb93-fcc4-47f2-8ceb-fd88f01ca17f (Retrieved 7th April, 2017).
- ↑ 12.0 12.1 12.2 PICILLO, Marina, FASANO, Alfonso. Recent advances in Essential Tremor: Surgical treatment. Parkinsonism & Related Disorders, 2016, 22(1), S171–S175. DOI: 10.1016/j.parkreldis.2015.09.012 Available online at: http://www.sciencedirect.com/science/article/pii/S1353802015003879 (Retrieved 6th April, 2017).
- ↑ 13.0 13.1 St. Jude Medical, Inc. Abbott Acquisition of St. Jude Medical Set to Close on January 4, 2017. Jude Medical, Inc. [online]. 2016, Dec 30. Available online at: http://media.sjm.com/newsroom/news-releases/news-releases-details/2016/Abbott-Acquisition-of-St-Jude-Medical-Set-to-Close-on-January-4-2017/default.aspx (Retrieved 7th April, 2017).
- ↑ Abbott Laboratories. Contacts. Abbott Laboratories [online]. Available online at: http://www.abbott.com/contact.html (Retrieved 7th April, 2017).
- ↑ Abbott Laboratories. Executive Team. Abbott Laboratories [online]. Available online at: http://www.abbott.com/corpnewsroom/utilities/executive-team.html (Retrieved 7th April, 2017).
- ↑ Abbott Laboratories. Michael T. Rousseau. Abbott Laboratories [online]. Available online at: http://www.abbott.com/corpnewsroom/utilities/executive-team/michael-rousseau.html (Retrieved 7th April, 2017).
- ↑ St. Jude Medical, Inc. Our History: Investing in People and Ideas. St. Jude Medical, Inc. [online]. Available online at: https://www.sjmglobal.com/en-int/about/company-facts/history?clset=92f57278-460e-4300-b7fe-89e52a04194f%3acadddb93-fcc4-47f2-8ceb-fd88f01ca17f (Retrieved 6th April, 2017).
- ↑ 18.0 18.1 National Institute of Neurological Disorders and Stroke. Parkinson's Disease Information Page. National Institute of Neurological Disorders and Stroke [online]. Available online at: https://www.ninds.nih.gov/Disorders/All-Disorders/Parkinsons-Disease-Information-Page (Retrieved 10th April, 2017).
- ↑ POLYMEROPOULOS, M. H. et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science [online]. 1997, Jun 27. 276(5321), 2045-2047. Available online at: http://ve5kj6kj8s.scholar.serialssolutions.com/?sid=google&auinit=MH&aulast=Polymeropoulos&atitle=Mutation+in+the+%CE%B1-synuclein+gene+identified+in+families+with+Parkinson%27s+disease&id=pmid:9197268 (Retrieved 10th April, 2017).
- ↑ National Institute of Neurological Disorders and Stroke. Essential Tremor Information Page. National Institute of Neurological Disorders and Stroke [online]. Available online at: https://www.ninds.nih.gov/Disorders/All-Disorders/Essential-Tremor-Information-Page (Retrieved 10th April, 2017).
- ↑ 21.0 21.1 National Institute of Neurological Disorders and Stroke. Dystonias Information Page. National Institute of Neurological Disorders and Stroke [online]. Available online at: https://www.ninds.nih.gov/Disorders/All-Disorders/Dystonias-Information-Page (Retrieved 10th April, 2017).
- ↑ Gillies, Martin J. et al. Rechargeable vs. Nonrechargeable Internal Pulse Generators in the Management of Dystonia. Neuromodulation: Technology at the Neural Interface, 2013, 16(3), 226-229. Doi: 10.1111/ner.12026 Available online at: http://onlinelibrary.wiley.com/doi/10.1111/ner.12026/full (Retrieved 11th April, 2017).
- ↑ St. Jude Medical, Inc. Important Medical Device Information. Gov.uk [online]. 2011, May 24. Available online at: https://assets.publishing.service.gov.uk/media/5485ac15e5274a42900002ab/con117559.pdf (Retrieved 11th April, 2017).
- ↑ MassDevice Staff. St. Jude yanks Brio deep brain stimulator. MassDevice [online]. 2012, Mar 29. Available online at: http://www.massdevice.com/st-jude-yanks-brio-deep-brain-stimulator/ (Retrieved 11th April, 2017).
- ↑ MCINTOSH, James. Parkinson's brain implant approved by FDA. Medical News Today [online]. 2015, Jun 15. Available online at: http://www.medicalnewstoday.com/articles/295376.php?trendmd-shared=0 (Retrieved 7th April, 2017).