RNS® System
| RNS® System | |
|---|---|

|
|
| Category | Deep brain stimulation |
| Developer | NeuroPace, Inc. Mountain View, California [1] |
| Announced | December, 2009 [2][3] |
| Released | Developers:
Consumers: 2013 |
| Price | 35,000 USD (estimated)[4] |
| Max output | 11.5 mA11.5 T 0.0115 A ± 10%, at 500 Ohms; [5] |
| Session duration | 55 s 0.0833 minute (min 10 msec)[5] |
| Scalp location | not settled location (in parietal skull upon recommendation) [5] |
| Weight | 18 g [5] |
| Controls |
laptop personal computer, magnet, NeuroPace Patient Data Management System, NeuroPace Programmer [5] |
| Data available | good |
| Risk factor | |
| Medical prescription | yes |
| http://www.neuropace.com/ | |
Responsive Neurostimulation System, RNS® System (hereinafter referred to as RNS System) is medical device for epilepsy treatment.[6] It can monitor and stimulate brain activity, for it is deep brain stimulation device. It is manufactured by privately held company named NeuroPace, which is located in Montain View, California.[7] The therapy with RNS System consists in reducing the frequency of seizures in individuals[5], who have partial-onset, medically refractory (drug resistant) epilepsy.[8] Those patients are treated with the RNS System, when they have no more than two epileptogenic foci (epileptogenic focus is the source or starting point of the seizures) and are resistant to more than two antiepileptic medications. The RNS System is thus only for medical prescription.
Contents
Main Characteristics
The RNS Neurostimulator monitors electrical activity in the brain via the implanted patient leads and delivers therapy in the form of electrical stimulation, when the seizures begin.
Plain device consist of:
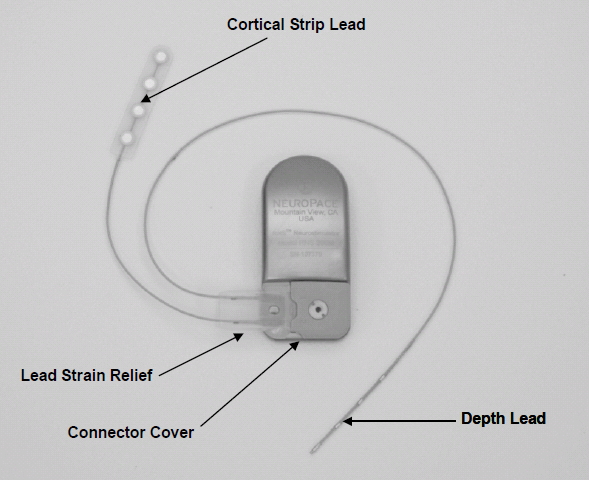
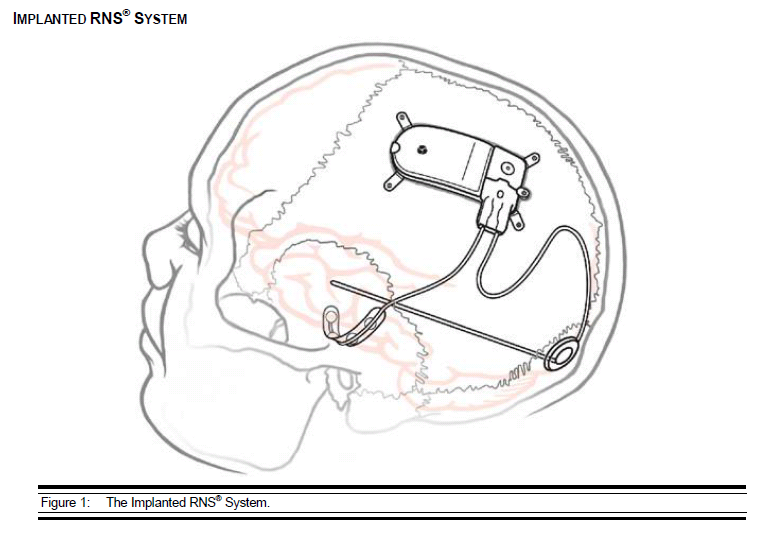
- RNS Neurostimulator, which is a responsive electrical stimulation medical device (battery powered and microprocessor controlled)[5] Responsive electrical stimulation is a new approach to treat epilepsy and RNS System is the first device to provide it.[9] RNS Neurostimulator records certain electrocorticographic (ECoG) registred patterns in the brain, which causes seizures. Then delivers short trains of current pulses through the leads to interrupt those patterns. Each stimulation can contain two bursts. Neurostimulator must be implanted in the skull together with leads.[5] The Neurostimulator should work for about 2 to 3.5 years, before battery power is drained. It is size of 28 x 60 mm, so it is relatively small device.[10]
- NeuroPace Leads are placed in the brain and are connected to Neurostimulator. One lead contains four electrodes. There are two kind of these leads. First, the Cortical Strip Leads are intended to subdural implant and are available in 15, 25 or 35 centimetres length. Four electrodes in leads are spaced 10 millimetres apart from each other. Second, the Depth Leads are intended for implant into brain. They are in four combinations - 30 centimetre length, 10 millimetre electrode spacing; 30 centimetre length, 3.5 millimetre electrode spacing; 44 centimetre length, 10 millimetre electrode spacing or 44 centimetre length, 3.5 millimetre electrode spacing.[5]
- NeuroPace components and accessories contain several devices, for example cranial prosthesis or magnet, which might be used as suppressor of therapy or starter an electrocorticogram storage (records ECoG patterns), if it is placed over the implanted RNS Neurostimulator.[5]
Patients also receive:
- NeuroPace Programmer, which contain a laptop personal computer with software developed by NeuroPace. It prescribe how the neurostimulator operates. Programmer might sets up recording ECoG patterns, perform detection analysis or communicate with NeuroPace Patient Data Management System (PDMS) via the Internet.[5]
- Telemetry Wand permits communication with the programmer via a USB cable.[5]
- NeuroPace Patient Data Management System (PDMS) contains obtained data from patient, who has access via the Internet. User must be authorized for using this system.[5]
Purpose
Main purpose of the RNS System is to treat medical condition called epilepsy. The goal of the RNS System is not enhance human´s cognitive capabilities.
Company & People
- Frank M. Fischer: the CEO of NeuroPace
- Rebecca L. Kuhn: Chief Financial Officer, Vice President, Finance & Administration, NeuroPace
- Martha J. Morrell, MD: Chief Medical Officer of NeuroPace
- Isabella R. Abati: Vice President, Regulatory Affairs, NeuroPace
- Debra L. Smolley: Vice President, Quality Assurance & Manufacturing Operations, NeuroPace[1]
Important Dates
- January 19, 2004: the first patient of the Feasibility Study[11]
- December 29, 2005: start of the Pivotal Study[11]
- April 6, 2007: start of the Long-term Treatment Study (LTT)[11]
- November 14, 2013: FDA Premarket Approval for the NeuroPace® RNS® System[12]
- May 2023: The completion of study for long time effect of the brain stimulation[13]
Enhancement/Therapy/Treatment
Enhancement
The RNS System is medical device to treat epilepsy. It is not designed for enhancing human capabilities, although it was demonstrated, that the RNS System has no adverse cognitive effect in epileptics.[14] In several cases it even comes to some improvements in the cognitive capabilities. Although these findings are inconclusive for they result from very various data.[15] A possible explanation of the alleged cognitive enhancement may rest in reduced seizures. But this is not enhancement of the sort, but rather the positive consequence of the successful treatment of epilepsy.
Epilepsy
Epilepsy or “seizure disorder” is a chronic disorder, which causes unpredictable seizures of neuronal activity. In epilepsy, the normal pattern of neuronal activity becomes disturbed, causing strange sensations, emotions, and behavior or sometimes convulsions, muscle spasms, and loss of consciousness. It might be caused by some diseases such as brain tumors, Alzheimer’s disease, brain damage, abnormal brain development, genetic mutation (de novo mutations) etc..[16] Epilepsy usually does not have a simple or known cause.[17] Epilepsy always arises from brain and is bound to sudden abnormal brain electrical activity.[16] Clusters of neurons might fire signal faster than normal, which is as many as 500 times a second. That electric activity then causes seizure. This medical condition is considered, when a person has at least two or more unprovoked seizures separated by 24 hours. In the United States suffer from epilepsies up to 2.3 million adults and more than 450,000 children.[16] Without satisfactory seizure control still remain 15-40% of all patients.[18]
There are many possible triggers of seizures in epilepsy.[16] The most common perceived triggers are: missing medication (40.9%), emotional stress (31.3%), sleep deprivation (19.7%), fatigue (15.3%), missing meals (9.1%), fever (6.4%), and smoking (6.4%). Data was obtained form 405 patients.[19] Only around 3% of epileptics have a photosensitive epilepsy. That is the kind of epilepsy, which is triggered by certain visual patterns, such as flashing lights.[20] There are more than 30 different types of seizures, but generally they are divided into the two major groups – the focal seizures, which affect only in one part of the brain, and the generalized seizures, which distorts the electrical activity of the whole or a larger portion of the brain. The seizures might look very different according to where it occurs. They might or might not cause loss of consciousness, or a muscle´s massive contractions. It might also appear, for example in the intense feeling of déjà vu (partial seizures in temporal lobe). Epilepsy has also many various types, for example the absence epilepsy (characterized by a brief loss and return of consciousness), the frontal lobe epilepsy, the temporal lobe epilepsy (most common form in focal seizures) or the neocortical epilepsy.[16] Epilepsy has also some risks associated with the life-threatening conditions, such as "status epilepticus" and "sudden unexpected death". However these cases are relatively rare. More common risks are appearing in connection with the injury during seizures.[16]
Treatment
The main treatment scenario of epilepsy is anticonvulsant medications (such as phenytoin, carbamazepine, lamotrigine and valproate), possibly for the person's entire life. The treatment of epilepsy may be also aided with a diet (a hight-fat, very low carbonhydrate ketogenic diet). A medication resistant epilepsy might be treated with surgery (only if focal seizures persist after at least two medications or if it is identifiable brain lesions) or devices just like the RNS System. There are some different possible devices to treat epilepsy, for instance, the Vagus nerve stimulator (approved by the FDA in 1997) or the experimental devices not approved by the FDA: the trigeminal nerve stimulation or the transcutaneous magnetic stimulation.[16]
The treatment with the RNS System provides responsive cortical stimulation via neurostimulator connected to depth or subdural cortical strip leads. They are placed in the brain based on seizure focus. The neurostimulator continually scans electrocorticographic activity, detects abnormal activity and provides stimulation. The physician regulates and optimizes the parameters for each patient individually.[21] It always depends upon where in the brain is the focus of epilepsy. Seizures might come from different cortical location, that include temporal, frontal, centroparietal, or occipital lobe regions.[22] But the RNS System is adjusted only for at the most two foci of seizures.[5]
So the treatment with the help of the RNS System is not intended for everyone. The patient must fulfil several conditions: he must have the focal (partial)[23] seizures, has average three or more disabling seizures per month (over the three most recent month), and he has to be resistant to the medications or diet (drug resistant is in the case of RNS System defined according to ILAE as a failure to control seizures after two seizures medications).
Ethical & Health Issues
Health Issues
The use of the RNS System is a relatively severe intervention in the brain and thus involves several health risks. Most of the risks are rather connected with a health issues than with a ethical issues.
- Risks
First of all there are some risks associate with surgery to implant the RNS Neurostimulator and Leads: post-implant infection (7%) and bleeding in the brain or under the skull because of the implant (4.7%). After about 2 or 3 years of using, the Neurostimulator has to be replaced with the new one, so the patient must get through another surgery. However the second one is not so dangerous, because the doctor will not have to do any surgery on the bone.[10]
The implanted devices also might negatively interact with another medical procedures, such as computed tomography (CT) or magnetic resonance (MRI).[10] It might also cause allergic reaction, skin erosion (around the Neurostimulator), lead to migration (move from their desired implant location) etc. There always remains a risk of failure the RNS devices.[10]
Serious risk might also come from unknown effect of long-term brain stimulation.[10] This conclusion supports as well the study conducted by Loring, Kapur, Meador and Morrell.[24] It is probably too soon to determine exact effect of the brain stimulation. The study for long time effect of the brain stimulation is still under way and the estimated completion date is May 2023.[13]
- Benefits
On the other hand, there are clear benefits of the RNS System. It was demonstrated that the RNS System significantly reduces epileptic seizures. According to clinical studies (Pivotal study – 191 subjects and Feasible study – 65 subjects), treatment patients come to the monthly reduction in seizure frequency averaging 37.9% compared to a 17.3% reduction in the sham (control) group.[10]
According to the FDA:
“Given the available information above [clinical studies], the data support that for the following indications for use the probable benefits outweigh the probable risks.”[10]
So it is clear that the RNS System is risky, but at the same time it helps people with epilepsy to reduce seizures. It still does not help every patient with epilepsy, because the RNS System is designed only to certain type of the epileptic condition. However according to Thomas and Jobst the RNS System might not be so effective in seizure reduction as epilepsy surgery.[8] On the other hand, epilepsy surgery is not always appropriate for certain type of epileptic illness. The RNS System thus remains suitable alternative to treat epilepsy. Similar conclusion also supports The University of Southern California (USC) Neurorestoration Centre and the Keck Hospital of USC, that are the world's first institutions to implant the RNS System post FDA approval. They confirm that:
"The RNS System can be readily incorporated into an active epilepsy surgical center."[25]
Ethical Issues
Some danger might arise also from hacker´s attack. The device interacts with the programmer, which is possible to replicate and then operate the Neurostimulator. But according to Frank Fisher, the CEO of the NeuroPace, it is very unlikely due to the fact, that it is significantly difficult and there is neither a reason for someone to do that, because the hacker has in theory only two options. One option is that he might do something with the device, so it was not function any more. Or he might deliver several inappropriate stimulation and cause a seizure.[26] These risks should not have been taken lightly, even if they are very unlikely.
In case of the RNS System is required to consider, if benefits outweigh considerable risks. According to FDA, they did. But it depends on each patient individually after all. Some people might have a problem with implementation a device in the brain, because every intervention to the brain is very risky. For some people it might be completely unacceptable that they would have the device in their bodies, for example, based on their beliefs.
Public & Media Impact and Presentation
There are many successful stories of patients with implanted RNS System. One of the first patient, who received the RNS System outside the study group was Tracey Drake in November 2014 at New York-Presbyterian/Weill Cornell Medical Center. She has reported very positive experience with the RNS System.[27] There are several similar stories that show positive effects of the RNS System.
For example Ian Olsen, who suffers from epilepsy since 11 years old, says about RNS System this: After dealing with seizures for 16 years, I feel like I have now defeated my seizures once and for all. The RNS system is helping more and more people with epilepsy – and everyone battling seizures should know about it.[28]
Now is also especially necessary to establish exactly which role the RNS System will play in treatment of epilepsy. In fact, the RNS System is still at the beginning and it is perceived more like alternative to AED (antiepileptic drug) or surgery.[15] There remains a question how will look the RNS System in the future. Frank Fischer note that:
It’s totally logical to me that any neurological condition that results in changes in brain state, where the brain state, by monitoring the brain state that can be determined and then treated by virtue of the delivery of stimulation, may well be able to be treated by virtue of this technology.[26]
Those neurological condition he means, for example, depression.
Public Policy
The RNS System was approved (Premarket Approval, PMA) by Food and Drug Administration (FDA) in 2013 based on clinical studies. Those contain a Feasibility study, a Pivotal study, and a Long-term Treatment study. There were also several studies in laboratory, where were tested every single device of the RNS System. Before human studies, there were also studies on animals, particularly on sheep. The Feasibility study contained 65 subjects and was aimed at preliminary safety and effectiveness. The results of the Feasibility study was used in the Pivotal study, which was performed "to establish a reasonable assurance of safety and effectiveness of the RNS System". It was containing 191 subjects in total. The Long-term Treatment study is still ongoing. It contains 230 subjects.[11]
The RNS System devices are connected with several patents. On the website of the NeuroPace company, there are list of the patents for every single device of RNS System: NeuroPace® RNS® Neurostimulator, NeuroPace® Depth Lead, NeuroPace® Cortical Strip Lead, NeuroPace® Programmer, NeuroPace® Remote Monitor, Wand and Ferrule.[29]
Related Technologies, Projects, or Scientific Research
There exist several possible treatment for epilepsy on the basis of deep brain stimulation: vagus nerve stimulation (VNS), trigeminal nerve stimulation (TNS), deep brain stimulation (DBS), and closed-loop stimulation system (RNS System).[18]
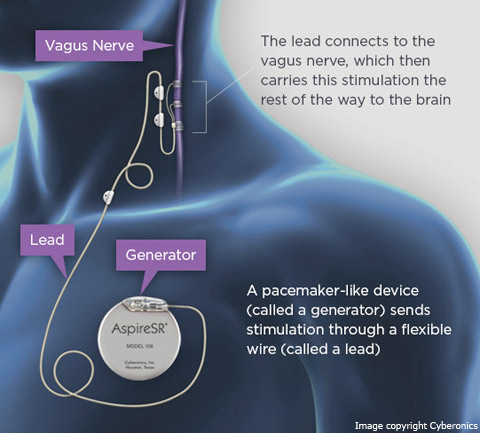
VNS, manufactured by Cyberonics, Inc.[30], sends regular, mild pules of electrical energy to the brain via the vagus nerve. It is placed under the skin in the chest and in the neck.[31] VNS began clinical investigation in 1988 and was approved by FDA (Premarket Approval) for the treatment of medically refractory epilepsy in 1997. VNS is intended to treat epilespy of partial-onset seizures. But recent studies have suggested to treat with VNS other epilepsy syndromes, such as idiopthic generalized epilepsies and Lennox-Gestaut syndrome. Efficacy of VNS in the seizures reduction is ranging between 35 - 50%. It always depend on the particularly study. VNS has been associated with positive improvement of alertness and mood (treatment-resistant depression). The negative side effects are connected with acute phase after implantation (intermittent hoarseness (28%), cough (14%), voice alteration (13%), paresthesias (12%), headache (4.5%), and shortness of breath (3.2%)).[18]
TNS is not yet been approved by FDA. It has been studied in animal and pilot clinical trials. But it has not yet been sufficiently corroborated, that TNS is effective to treat epilepsy, based on relatively small scale of the trials.[32]
DBS treatment is stimulation of the different areas of central nervous system. DBS has been applied in a different brain areas (anterior nucleus of the thalamus, cerebellum, hippocampus, subthalamic nucleus or nucleus accumbens) according to the clinical indications. The DBS stimulation has been approved first by European Union (Conformité Européenne, CE) for company Medtronic in 2013 [33], and than by FDA in 2013 for treatment of epilepsy (under the FDAs Humanitarian Device Exception)[34]. Effectiveness of the stimulation in the anterior nucleus is estimated on 50% reduction seizures.[18] DBS therapy is also currently approved for the treatment another neuropsychiatric disorders such as essential tremor, advanced Parkinson's disease, dystonia and treatment-resistant obsessive-compulsive disorder (OCD).
The RNS System is on the other hand based on closed-loop stimulation, which provide stimulation only if triggered by early seizure activity. However the above mentioned systems of stimulation provide stimulation constantly, regardless of neuronal activity. So the RNS System has less adverse effect than the rest.[18]
There are also some similar devices, that treat the different kind of diseases. One of them is, for example the Activa Deep Brain Stimulation Therapy System (Medtronic), which is target to treat tremor associated with Parkinson´s disease and essential tremor. It was approved by FDA in 1997.[35] Similar device for reducing the symptoms of Parkinson's disease is the Brio™ Rechargeable IPG, which was approved by FDA in 2015.[36]
References
- ↑ 1.0 1.1 NeuroPace. Company Overview. NeuroPace, Inc. [online]. © 2016. Available online at: http://www.neuropace.com/about-us-corporate/ (Retrieved 4.11.2016).
- ↑ NOVELLI, P. NeuroPace announces clinical trial results demonstrating the RNS System. News medical life sciences [online]. 2009, Dec 9. Available online at: http://www.news-medical.net/news/20091209/NeuroPace-announces-clinical-trial-results-demonstrating-the-RNS-System.aspx (Retrieved 4.11.2016).
- ↑ Pivotal Trial Data Demonstrate NeuroPace RNS® System Reduced Seizures in People with Epilepsy. NeuroPace, Inc. [online]. © 2016. Available online at: http://www.neuropace.com/pivotal-trial-data-demonstrate-neuropace/ (Retrieved 4.11.2016).
- ↑ TRINIDAD, T. Keck Medicine physicians first to implant epilepsy-controlling device. USC News [online]. 2013, Dec 19. Available online at: https://news.usc.edu/57947/keck-medicine-physicians-become-first-to-implant-epilepsy-controlling-device/ (Retrieved 4.11.2016).
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 NeuroPace. RNS® System User Manual. NeuroPace, Inc. [online]. © 2016. Available online at: http://www.neuropace.com/wp-content/uploads/2016/08/RNS_System_User_Manual.pdf (Retrieved 4.11.2016).
- ↑ NeuroPace. About the technology. NeuroPace, Inc. [online]. © 2016. Available online at: http://www.neuropace.com/the-rns-system/ (Retrieved 4.11.2016).
- ↑ NeuroPace RNS® System Honored With Prestigious Award At The 22nd Annual Phoenix Conference. Business Wire: A Berkshire Hathaway Company [online]. 2015, Nov 4. Available online at: http://www.businesswire.com/news/home/20151104005381/en/NeuroPace-RNS%C2%AESystem-Honored-Prestigious-Award-22nd (Retrieved 4.11.2016).
- ↑ 8.0 8.1 THOMAS, G.P., JOBST, B.C. Critical review of the responsive neurostimulator system for epilepsy. The National Center for Biotechnology Information [online]. 2015, Oct 1. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4598207/ (Retrieved 4.11.2016).
- ↑ SIRVEN, J.I. Responsive Neurostimulation. Epilepsy Foundation [online]. 2014, May. Available online at: http://www.epilepsy.com/learn/treating-seizures-and-epilepsy/devices/responsive-neurostimulation (Retrieved 4.11.2016).
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 NeuroPace. NeuroPace® RNS® System Patient Manual. NeuroPace, Inc [online]. © 2016. Available online at: http://www.accessdata.fda.gov/cdrh_docs/pdf10/P100026C.pdf (Retrieved 4.11.2016).
- ↑ 11.0 11.1 11.2 11.3 U.S. Food & Drug Administration. Summary of Safety and Effectiveness Data (SSED). U.S. Food & Drug Administration [online]. 2013. Available online at: http://www.accessdata.fda.gov/cdrh_docs/pdf10/P100026B.pdf (Retrieved 4.11.2016).
- ↑ U.S. Food and Drug Administration. FDA Grants Premarket Approval (PMA) for the NeuroPace® RNS® System to treat Medically Refractory Epilepsy. NeuroPace, Inc. [online]. 2013, Nov. Available online at: http://www.neuropace.com/wp-content/uploads/2015/11/NeuroPace_Press_Release_PMA_Approval_2013-11-14.pdf(Retrieved 6.11.2016
- ↑ 13.0 13.1 RNS® System Epilepsy PAS. ClinicalTrials.gov [online]. 2016, Sep. Available online at: https://clinicaltrials.gov/ct2/show/results/NCT02403843 (Retrieved 6.11.2016).
- ↑ NeuroPace RNS® System Associated with Positive Effects In Memory and Language for People Living with Partial Onset Epilepsy. Business Wire: A Berkshire Hathaway Company [online]. 2015, Oct 20. Available online at: http://www.businesswire.com/news/home/20151020005454/en/NeuroPace-RNS%C2%AE-System-Positive-Effects-Memory-Language (Retrieved 4.11.2016).
- ↑ 15.0 15.1 SPENCER, D. Responsive Neurostimulation and Cognition. Epilepsy Currents [online]. 2016, March-April, Vol. 16, No. 2, pp. 98-100. Available online at: http://www.epilepsycurrents.org/doi/full/10.5698/1535-7511-16.2.98 (Retrieved 4.11.2016).
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 National Institute of Neurological Disorders and Stroke. The Epilepsies and Seizures. National Institute of Neurological Disorders and Stroke [online]. 2015, Aug, NIH Publication No. 15-156. Available online at: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Epilepsies-and-Seizures-Hope-Through (Retrieved 27.04.2017).
- ↑ SIRVEN, J.I. What Is Epilepsy? Epilepsy Foundation [online]. 2014, Jan. Available online at: http://www.epilepsy.com/learn/epilepsy-101/what-epilepsy (Retrieved 4.11.2016).
- ↑ 18.0 18.1 18.2 18.3 18.4 WU, C., SHARAN, A.D. Neurostimulation for the Treatment of Epilepsy: A Review of Current Surgical Interventions. Neuromodulation, 2013, 16, 10–24. Doi: 10.1111/j.1525-1403.2012.00501.x Available online at: http://onlinelibrary.wiley.com/doi/10.1111/j.1525-1403.2012.00501.x/abstract (Retrieved 13th April, 2017).
- ↑ BALAMURUGAN, et al. Perceived trigger factors of seizures in persons with epilepsy. Elsevier Inc., 2013, Vol. 33, Iss. 9, pp 743 - 747. Available online at: http://www.seizure-journal.com/article/S1059-1311(13)00177-5/pdf (Retrieved 4.11.2016).
- ↑ SHAFER, P.O., SIRVEN, J.I. Photosensitivity and Seizures. Epilepsy foundation [online]. 2013, Nov. Available online at: http://www.epilepsy.com/learn/triggers-seizures/photosensitivity-and-seizures (Retrieved 5.12.2016).
- ↑ MORRELL, M.J. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. Neurology, 2011, Sep 27, vol. 77, no. 13, pp. 1275-1304. ISSN 0028-3878.
- ↑ PANAYIOTOPOULOS, C.P. Familial Focal Epilepsy with Variable Foci. Epilepsy Foundation [online]. 2005, Jan. Available online at: http://www.epilepsy.com/information/professionals/about-epilepsy-seizures/familial-autosomal-dominant-focal-epilepsies-2 (Retrieved 4.11.2016).
- ↑ According to International League Against Epilepsy (ILAE) is the concept of “partial” replaced by “focal” to unified terminology of epilepsies
- ↑ LORING, D.W., KAPUR, R., MEADOR, K.J., MORRELL, M.J. Differential neuropsychological outcomes following targeted responsive neurostimulation for partial-onset epilepsy. Epilepsia [online], 2015, Vol. 56, No. 11, pp. 1836–1844. Available online at: http://onlinelibrary.wiley.com/doi/10.1111/epi.13191/epdf (Retrieved 6.11.2016).
- ↑ LEE, B., ZUBAIR, M.N., MARQUEZ, Y.D., LEE, D.M., KALAYJIAN, L.A., HECK, C.N., LIU, C.Y. A Single-Center Experience with the NeuroPace RNS System: A Review of Techniques and Potential Problems. Elsevier Inc., 2015, Vol. 84, Iss. 3, pp. 719 - 726. Available online at: http://www.worldneurosurgery.org/article/S1878-8750(15)00479-9/references (Retrieved 7.11.2016).
- ↑ 26.0 26.1 NeuroPace: Controlling Epilepsy With a Brain Implant. Interview with Frank Fischer, the CEO of NeuroPace, Inc. IEEE Spectrum [online]. 2013, Nov 5. Available online at: http://spectrum.ieee.org/podcast/biomedical/bionics/neuropace-controlling-epilepsy-with-a-brain-implant (Retrieved 6.11.2016).
- ↑ Epilepsy Team Implants First NeuroPace RNS Neurostimulator. Weill Cornell Brain and Spine Center [online]. 2014, Nov 7. Available online at: http://weillcornellbrainandspine.org/in-the-news/epilepsy-team-implants-first-neuropace-rns-neurostimulator (Retrieved 6.11.2016)
- ↑ NeuroPace. Ian’s Story. NeuroPace, Inc. [online]. © 2016. Available online at: http://www.neuropace.com/ians-story/ (Retrieved 30.11.2016).
- ↑ NeuroPace. Legal Terms. NeuroPace, Inc. [online]. © 2016. Available online at: http://www.neuropace.com/legal/ (Retrieved 30.11.2016).
- ↑ U.S. Food & Drug Administration. Premarket Approval (VNS Therapy System). U.S. Food & Drug Administration [online]. 2016. Available online at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P970003 (Retrieved 6.12.2016).
- ↑ SCHACHTER, S.C., SIRVEN, J.I. Vagus Nerve Stimulation (VNS). Epilepsy foundation [online]. 2013. Available online at: http://www.epilepsy.com/learn/treating-seizures-and-epilepsy/devices/vagus-nerve-stimulation-vns (Retrieved 2.12.2016).
- ↑ PACK, A.M. Trigeminal Nerve Stimulation May Not Be Effective for the Treatment of Refractory Partial Seizures. Epilepsy Currents. 2013, Vol. 13, No. 4, pp. 164–165.
- ↑ Medtronic. Medtronic Receives European CE Mark Approval for Deep Brain Stimulation Therapy for Refractory Epilepsy. Medtronic, Inc. [online]. 2010. Available online at: http://newsroom.medtronic.com/phoenix.zhtml?c=251324&p=irol-newsArticle&ID=1773303 (Retrieved 5.12.2016).
- ↑ TEKRIWAL, A., BALTUCH, G. Deep Brain Stimulation: Expanding Applications. Neurol Med Chir, Tokyo, 2015, Vol. 55, No. 12, pp. 861–877.
- ↑ JEFFREY, S. FDA Okays Brio Neurostimulation System for PD, ET. Medscape [online]. 2015, Jun 12. Available online at: http://www.medscape.com/viewarticle/846456 (Retrieved 3.12.2016).
- ↑ U.S. Food & Drug Administration. FDA approves brain implant to help reduce Parkinson’s disease and essential tremor symptoms. U.S. Food & Drug Administration: FDA News Release [online]. 2015, Jun 12. Available online at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm451152.htm (Retrieved 3.12.2016).